TGF-β Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation
- PMID: 28108485
- PMCID: PMC5495062
- DOI: 10.1101/cshperspect.a022186
TGF-β Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation
Abstract
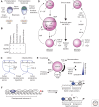
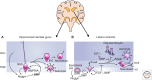
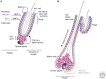
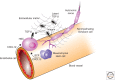
Soon after the discovery of transforming growth factor-β (TGF-β), seminal work in vertebrate and invertebrate models revealed the TGF-β family to be central regulators of tissue morphogenesis. Members of the TGF-β family direct some of the earliest cell-fate decisions in animal development, coordinate complex organogenesis, and contribute to tissue homeostasis in the adult. Here, we focus on the role of the TGF-β family in mammalian stem-cell biology and discuss its wide and varied activities both in the regulation of pluripotency and in cell-fate commitment.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Ahrens M, Ankenbauer T, Schröder D, Hollnagel A, Mayer H, Gross G. 1993. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol 12: 871–880. - PubMed
-
- Aizawa K, Ageyama N, Terao K, Hisatsune T. 2011. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging 32: 140–150. - PubMed
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. 2000. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227: 271–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
