Transition Zone Migration: A Mechanism for Cytoplasmic Ciliogenesis and Postaxonemal Centriole Elongation
- PMID: 28108487
- PMCID: PMC5538411
- DOI: 10.1101/cshperspect.a028142
Transition Zone Migration: A Mechanism for Cytoplasmic Ciliogenesis and Postaxonemal Centriole Elongation
Abstract
The cilium is an elongated and continuous structure that spans two major subcellular domains. The cytoplasmic domain contains a short centriole, which serves to nucleate the main projection of the cilium. This projection, known as the axoneme, remains separated from the cytoplasm by a specialized gatekeeping complex within a ciliary subdomain called the transition zone. In this way, the axoneme is compartmentalized. Intriguingly, however, this general principle of cilium biology is altered in the sperm cells of many animals, which instead contain a cytoplasmic axoneme domain. Here, we discuss the hypothesis that the formation of specialized sperm giant centrioles and cytoplasmic cilia is mediated by the migration of the transition zone from its typical location as part of a structure known as the annulus and examine the intrinsic properties of the transition zone that may facilitate its migratory behavior.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
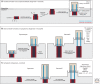


References
-
- Afzelius BA. 1955. The fine structure of the sea urchin spermatozoa as revealed by the electron microscope. Z Zellforsch Mikrosk Anat 42: 134–148. - PubMed
-
- Al-Dokhi O, Al-Onazee Y, Mubarak M. 2007. Fine structure of the epididymal sperm of the snake Eryx jayakari (Squamata, Reptilia). Int J Zool Res 3: 1–13.
-
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. 2004. Decoding cilia function: Defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539. - PubMed
-
- Baccetti B. 1982. The evolution of the sperm tail. Symp Soc Exp Biol 35: 521–532. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
