Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells
- PMID: 28108519
- PMCID: PMC5354955
- DOI: 10.1158/0008-5472.CAN-16-1645
Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells
Abstract
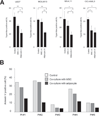
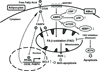
Leukemia cells in the bone marrow must meet the biochemical demands of increased cell proliferation and also survive by continually adapting to fluctuations in nutrient and oxygen availability. Thus, targeting metabolic abnormalities in leukemia cells located in the bone marrow is a novel therapeutic approach. In this study, we investigated the metabolic role of bone marrow adipocytes in supporting the growth of leukemic blasts. Prevention of nutrient starvation-induced apoptosis of leukemic cells by bone marrow adipocytes, as well as the metabolic and molecular mechanisms involved in this process, was investigated using various analytic techniques. In acute monocytic leukemia (AMoL) cells, the prevention of spontaneous apoptosis by bone marrow adipocytes was associated with an increase in fatty acid β-oxidation (FAO) along with the upregulation of PPARγ, FABP4, CD36, and BCL2 genes. In AMoL cells, bone marrow adipocyte coculture increased adiponectin receptor gene expression and its downstream target stress response kinase AMPK, p38 MAPK with autophagy activation, and upregulated antiapoptotic chaperone HSPs. Inhibition of FAO disrupted metabolic homeostasis, increased reactive oxygen species production, and induced the integrated stress response mediator ATF4 and apoptosis in AMoL cells cocultured with bone marrow adipocytes. Our results suggest that bone marrow adipocytes support AMoL cell survival by regulating their metabolic energy balance and that the disruption of FAO in bone marrow adipocytes may be an alternative, novel therapeutic strategy for AMoL therapy. Cancer Res; 77(6); 1453-64. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502(7472):489–498. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

