RNA-binding specificity landscape of the pentatricopeptide repeat protein PPR10
- PMID: 28108520
- PMCID: PMC5340921
- DOI: 10.1261/rna.059568.116
RNA-binding specificity landscape of the pentatricopeptide repeat protein PPR10
Abstract
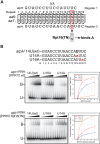
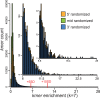
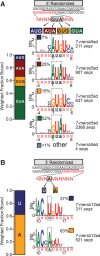
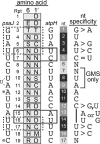
Pentatricopeptide repeat (PPR) proteins comprise a large family of helical repeat proteins that influence gene expression in mitochondria and chloroplasts. PPR tracts can bind RNA via a modular one repeat-one nucleotide mechanism in which the nucleotide is specified by the identities of several amino acids in each repeat. This mode of recognition, the so-called PPR code, offers opportunities for the prediction of native PPR binding sites and the design of proteins to bind specified RNAs. However, a deep understanding of the parameters that dictate the affinity and specificity of PPR-RNA interactions is necessary to realize these goals. We report a comprehensive analysis of the sequence specificity of PPR10, a protein that binds similar RNA sequences of ∼18 nucleotides (nt) near the chloroplast atpH and psaJ genes in maize. We assessed the contribution of each nucleotide in the atpH binding site to PPR10 affinity in vitro by analyzing the effects of single-nucleotide changes at each position. In a complementary approach, the RNAs bound by PPR10 from partially randomized RNA pools were analyzed by deep sequencing. The results revealed three patches in which nucleotide identity has a major impact on binding affinity. These include 5 nt for which protein contacts were not observed in a PPR10-RNA crystal structure and 4 nt that are not explained by current views of the PPR code. These findings highlight aspects of PPR-RNA interactions that pose challenges for binding site prediction and design.
Keywords: RNA-binding protein; bind-n-seq; chloroplast; helical repeat protein.
© 2017 Miranda et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
