Plasmodium Sporozoite Biology
- PMID: 28108531
- PMCID: PMC5411682
- DOI: 10.1101/cshperspect.a025478
Plasmodium Sporozoite Biology
Abstract
Plasmodium sporozoite transmission is a critical population bottleneck in parasite life-cycle progression and, hence, a target for prophylactic drugs and vaccines. The recent progress of a candidate antisporozoite subunit vaccine formulation to licensure highlights the importance of sporozoite transmission intervention in the malaria control portfolio. Sporozoites colonize mosquito salivary glands, migrate through the skin, penetrate blood vessels, breach the liver sinusoid, and invade hepatocytes. Understanding the molecular and cellular mechanisms that mediate the remarkable sporozoite journey in the invertebrate vector and the vertebrate host can inform evidence-based next-generation drug development programs and immune intervention strategies.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
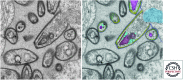
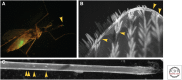



References
-
- Al-Olayan AM, Beetsma AL, Butcher GA, Sinden RE, Hurd H. 2002. Complete development of mosquito phases of malaria parasite in vitro. Science 295: 677–679. - PubMed
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Ménard R. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 12: 220–224. - PubMed
-
- Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prévost MC, Ishino T, Yuda M, Ménard R. 2008. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3: 88–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
