Cell Biology and Pathophysiology of α-Synuclein
- PMID: 28108534
- PMCID: PMC5519445
- DOI: 10.1101/cshperspect.a024091
Cell Biology and Pathophysiology of α-Synuclein
Abstract
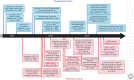
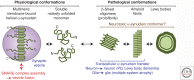
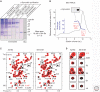
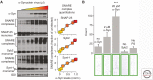
α-Synuclein is an abundant neuronal protein that is highly enriched in presynaptic nerve terminals. Genetics and neuropathology studies link α-synuclein to Parkinson's disease (PD) and other neurodegenerative disorders. Accumulation of misfolded oligomers and larger aggregates of α-synuclein defines multiple neurodegenerative diseases called synucleinopathies, but the mechanisms by which α-synuclein acts in neurodegeneration are unknown. Moreover, the normal cellular function of α-synuclein remains debated. In this perspective, we review the structural characteristics of α-synuclein, its developmental expression pattern, its cellular and subcellular localization, and its function in neurons. We also discuss recent progress on secretion of α-synuclein, which may contribute to its interneuronal spread in a prion-like fashion, and describe the neurotoxic effects of α-synuclein that are thought to be responsible for its role in neurodegeneration.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. 2000. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252. - PubMed
-
- Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OM. 2007. γ-Synuclein and the progression of cancer. FASEB J 21: 3419–3430. - PubMed
-
- Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, et al. 2002. α-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J Biol Chem 277: 12334–12342. - PubMed
-
- Ahn KJ, Paik SR, Chung KC, Kim J. 2006a. Amino acid sequence motifs and mechanistic features of the membrane translocation of α-synuclein. J Neurochem 97: 265–279. - PubMed
-
- Ahn M, Kim S, Kang M, Ryu Y, Kim TD. 2006b. Chaperone-like activities of α-synuclein: α-synuclein assists enzyme activities of esterases. Biochem Biophys Res Commun 346: 1142–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
