Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice
- PMID: 28108608
- PMCID: PMC5248991
- DOI: 10.2337/db16-0500
Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice
Abstract
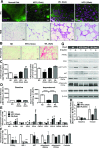
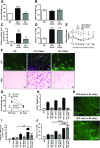
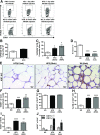
Obesity causes dramatic proinflammatory changes in the adipose tissue immune environment, but relatively little is known regarding how this inflammation responds to weight loss (WL). To understand the mechanisms by which meta-inflammation resolves during WL, we examined adipose tissue leukocytes in mice after withdrawal of a high-fat diet. After 8 weeks of WL, mice achieved similar weights and glucose tolerance values as age-matched lean controls but showed abnormal insulin tolerance. Despite fat mass normalization, total and CD11c+ adipose tissue macrophage (ATM) content remained elevated in WL mice for up to 6 months and was associated with persistent fibrosis in adipose tissue. ATMs in formerly obese mice demonstrated a proinflammatory profile, including elevated expression of interferon-γ, tumor necrosis factor-α, and interleukin-1β. T-cell-deficient Rag1-/- mice showed a degree of ATM persistence similar to that in WT mice, but with reduced inflammatory gene expression. ATM proliferation was identified as the predominant mechanism by which ATMs are retained in adipose tissue with WL. Our study suggests that WL does not completely resolve obesity-induced ATM activation, which may contribute to the persistent adipose tissue damage and reduced insulin sensitivity observed in formerly obese mice.
© 2017 by the American Diabetes Association.
Figures





References
-
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

