Loss of endothelial cell-specific molecule 1 promotes the tumorigenicity and metastasis of prostate cancer cells through regulation of the TIMP-1/MMP-9 expression
- PMID: 28108731
- PMCID: PMC5355147
- DOI: 10.18632/oncotarget.14684
Loss of endothelial cell-specific molecule 1 promotes the tumorigenicity and metastasis of prostate cancer cells through regulation of the TIMP-1/MMP-9 expression
Abstract
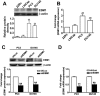
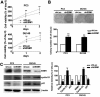
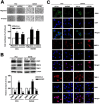
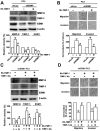
The Endothelial cell specific molecule-1 (ESM1) protein has been involved in proliferation and metastatic progression in multiple tumors. However, there are no studies regarding the mechanism of ESM1 in prostate cancer. We found that ESM1 knockdown in prostate cancer cells resulted in increased cell proliferation and colony formation ability response evidenced by decreased expression of p21 and increased expression of cyclin D1 in prostate cancer cells. Moreover, we revealed that knockdown ESM1 also induced the epithelial-mesenchymal transition (EMT), motility and invasiveness in accordance with the upregulated the MMP-9 expression, while downregulated the TIMP-1 expression. Recombinant human (Rh) TIMP-1 significantly attenuated ESM1-mediated cell migration and invasion. Additionally, ESM1 knockdown increased in vivo tumorigenicity and metastasis of prostate cancer cells. These findings provide the first evidence that the imbalance of MMP-9/TIMP-1, is one of the regulation mechanisms by which ESM1 promotes tumorigenicity and metastasis of prostate cancer cells.
Keywords: endothelial cell specific molecule 1; epithelial-mesenchymal transition; metastasis; prostate cancer cells; tumorigenicity.
Conflict of interest statement
None.
Figures


References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. - PubMed
-
- Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC. Family history and the risk of prostate cancer. Prostate. 1990;17:337–347. - PubMed
-
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17:292–306. - PubMed
-
- Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

