Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials
- PMID: 28108885
- PMCID: PMC5398984
- DOI: 10.1007/s13311-016-0507-6
Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials
Abstract
Radiographic endpoints including response and progression are important for the evaluation of new glioblastoma therapies. The current RANO criteria was developed to overcome many of the challenges identified with previous guidelines for response assessment, however, significant challenges and limitations remain. The current recommendations build on the strengths of the current RANO criteria, while addressing many of these limitations. Modifications to the current RANO criteria include suggestions for volumetric response evaluation, use contrast enhanced T1 subtraction maps to increase lesion conspicuity, removal of qualitative non-enhancing tumor assessment requirements, use of the post-radiation time point as the baseline for newly diagnosed glioblastoma response assessment, and "treatment-agnostic" response assessment rubrics for identifying pseudoprogression, pseudoresponse, and a confirmed durable response in newly diagnosed and recurrent glioblastoma trials.
Keywords: GBM; Glioblastoma; RANO; Response Assessment; T1 Subtraction.
Figures
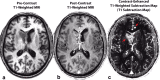
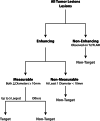


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

