Knockdown of TACC3 Inhibits the Proliferation and Invasion of Human Renal Cell Carcinoma Cells
- PMID: 28109075
- PMCID: PMC7844598
- DOI: 10.3727/096504017X14837020772250
Knockdown of TACC3 Inhibits the Proliferation and Invasion of Human Renal Cell Carcinoma Cells
Abstract
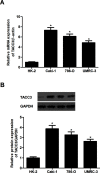
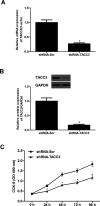
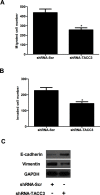
Transforming acidic coiled-coil protein 3 (TACC3) is a member of the TACC family and plays an important role in regulating cell mitosis, transcription, and tumorigenesis. However, the expression pattern and roles of TACC3 in renal cell carcinoma (RCC) remain unclear. The aim of this study was to investigate the role of TACC3 in RCC. We demonstrated overexpression of TACC3 in human RCC cell lines at both RNA and protein levels. Moreover, knockdown of TACC3 repressed RCC cell proliferation, migration, and invasion in vitro. In addition, knockdown of TACC3 inactivated PI3K/Akt signaling in RCC cells. Furthermore, knockdown of TACC3 significantly reduced tumor growth in xenograft tumor-bearing mice. Taken together, our findings showed that TACC3 was increased in human RCC cell lines, and knockdown of TACC3 inhibited the ability of cell proliferation, migration, invasion, and tumorigenesis in vivo. Therefore, TACC3 may act as a therapeutic target for the treatment of human RCC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
miRNA‑205‑5p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells.Oncol Rep. 2019 Nov;42(5):1677-1688. doi: 10.3892/or.2019.7307. Epub 2019 Sep 10. Oncol Rep. 2019. PMID: 31545453 Free PMC article.
-
Tuftelin 1 (TUFT1) Promotes the Proliferation and Migration of Renal Cell Carcinoma via PI3K/AKT Signaling Pathway.Pathol Oncol Res. 2021 Apr 19;27:640936. doi: 10.3389/pore.2021.640936. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34257606 Free PMC article.
-
Long non‑coding RNA MALAT1 correlates with cell viability and mobility by targeting miR‑22‑3p in renal cell carcinoma via the PI3K/Akt pathway.Oncol Rep. 2019 Feb;41(2):1113-1121. doi: 10.3892/or.2018.6853. Epub 2018 Nov 6. Oncol Rep. 2019. PMID: 30431104
-
Knockdown of TACC3 inhibits trophoblast cell migration and invasion through the PI3K/Akt signaling pathway.Mol Med Rep. 2016 Oct;14(4):3437-42. doi: 10.3892/mmr.2016.5659. Epub 2016 Aug 19. Mol Med Rep. 2016. PMID: 27572091
-
TACC3: a multi-functional protein promoting cancer cell survival and aggressiveness.Cell Cycle. 2023 Dec-Dec;22(23-24):2637-2655. doi: 10.1080/15384101.2024.2302243. Epub 2024 Jan 10. Cell Cycle. 2023. PMID: 38197196 Free PMC article. Review.
Cited by
-
Functional Assessment of Four Novel Immune-Related Biomarkers in the Pathogenesis of Clear Cell Renal Cell Carcinoma.Front Cell Dev Biol. 2021 Mar 16;9:621618. doi: 10.3389/fcell.2021.621618. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33796525 Free PMC article.
-
Inhibiting of TACC3 Promotes Cell Proliferation, Cell Invasion and the EMT Pathway in Breast Cancer.Front Genet. 2021 Jun 3;12:640078. doi: 10.3389/fgene.2021.640078. eCollection 2021. Front Genet. 2021. PMID: 34149795 Free PMC article.
-
TACC3 as an independent prognostic marker for solid tumors: a systematic review and meta-analysis.Oncotarget. 2017 Aug 24;8(43):75516-75527. doi: 10.18632/oncotarget.20466. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088887 Free PMC article.
-
TACC3 is a prognostic biomarker for kidney renal clear cell carcinoma and correlates with immune cell infiltration and T cell exhaustion.Aging (Albany NY). 2021 Mar 10;13(6):8541-8562. doi: 10.18632/aging.202668. Epub 2021 Mar 10. Aging (Albany NY). 2021. PMID: 33714201 Free PMC article.
-
Development and Validation of a Prognostic Gene Signature in Clear Cell Renal Cell Carcinoma.Front Mol Biosci. 2021 Apr 8;8:609865. doi: 10.3389/fmolb.2021.609865. eCollection 2021. Front Mol Biosci. 2021. PMID: 33968978 Free PMC article.
References
-
- Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, Sinescu IC. Renal cell carcinoma guideline. Actas Urol Esp. 2009;33:270–9. - PubMed
-
- Deveson KS. Renal cell carcinoma: Treatment options. Br J Nurs. 2011;20:536, 538–9. - PubMed
-
- Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, Eisen T, Horwich A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:40–8. - PubMed
-
- Hagan K, Trapp JD, Rhamy RK, Reynolds VH. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharm. 2009;67:1175–8. - PubMed
-
- Drucker BJ. Renal cell carcinoma: Current status and future prospects. Cancer Treat Rev. 2005;31:536–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
