Evolving prevalence of haematological malignancies in orphan designation procedures in the European Union
- PMID: 28109318
- PMCID: PMC5251220
- DOI: 10.1186/s13023-017-0567-7
Evolving prevalence of haematological malignancies in orphan designation procedures in the European Union
Abstract
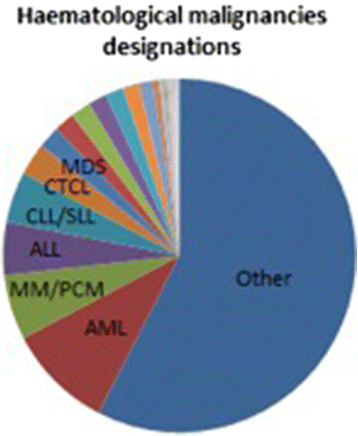
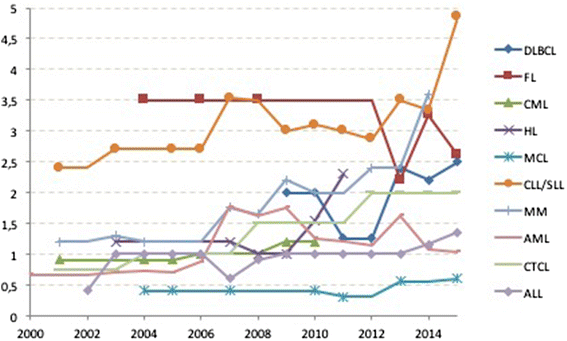
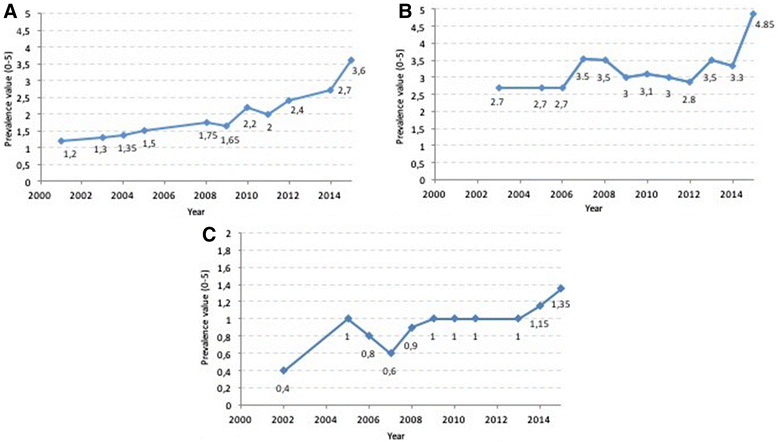
The Committee for Orphan Medicinal Products (COMP) evaluates prevalence of rare conditions as one of the criteria for granting an orphan designation with a prevalence threshold of 5 in 10.000. At the time of Marketing Authorisation (MA) these criteria are reassessed to ensure they are still met. The COMP has noted discordance between the prevalence of certain haematological malignancies at the time of Orphan Designation and at the time of Marketing Authorisation. Consequently, we conducted a retrospective assessment of Chronic Lymphocytic Lymphoma and Multiple Myeloma/Plasma cell Myeloma as well as several other haematological rare aetiologies frequently subject of orphan designation. These were: Diffuse large B-Cell Lymphoma (DLBCL), Follicular Lymphoma (FL), Cutaneous T-Cell Lymphoma (CTCL), Mantle Cell Lymphoma (MCL) and Chronic Myeloid Leukaemia (CML). The review used submissions as well as recent publications and results from external and EMA databases. As a first step in the analysis, an increase over time in the number of people affected was evident for four conditions in the COMP designation documents, whereas for DLBCL, FL, CTCL and MCL there had been no significant change, since the introduction of the Regulation in 2000. Specifically, the prevalence estimates increased from 1.2 to 3.6 per 10,000 for multiple myeloma, from 0.4 to 1.7 in acute lymphoblastic leukaemia, and from 2.7 to 4.85 for chronic lymphocytic leukaemia/small lymphocytic leukaemia and 1 to 2 in 10,000 for chronic myeloid leukaemia. The reasons for the changes in the prevalence of these four haematological conditions over the last 15 years were not assessed but recent publications have alluded to better outcomes due to new treatments being made available. In addition, many orphan diseases have a median age of onset over 60 years so that also the aging of the population may be a relevant contributing factor.
Keywords: Committee for orphan medicinal products; European orphan regulation; Haematological malignancies; Market exclusivity; Orphan designation; Prevalence.
Figures
References
-
- Opinions for Rare disease (orphan) designations. Eur Med Agency, [Online]. Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/orph.... Accessed 21 Sept 2016
-
- Survival of cancer patients in Europe. Eurocare, [Online]. Available: http://www.eurocare.it/. Accessed 25 Aug 2016
-
- Statistics for haematological malignancies. Haematol Malig Res Netw, [Online]. Available: http://www.hmrn.org/statistics. Accessed 25 Aug 2016
-
- COMP: Agendas, minutes and meeting reports. Eur Med Agency, [Online]. Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/docume.... Accessed 21 Sept 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

