Retinal oxygen: from animals to humans
- PMID: 28109737
- PMCID: PMC5441959
- DOI: 10.1016/j.preteyeres.2017.01.003
Retinal oxygen: from animals to humans
Abstract
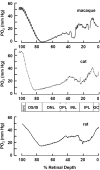
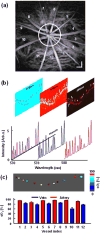
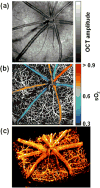
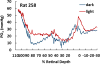
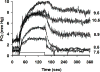
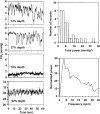
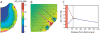
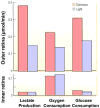
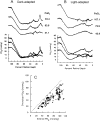
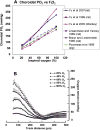
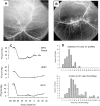
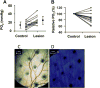
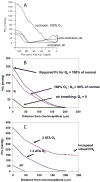
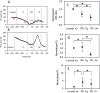
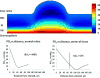
This article discusses retinal oxygenation and retinal metabolism by focusing on measurements made with two of the principal methods used to study O2 in the retina: measurements of PO2 with oxygen-sensitive microelectrodes in vivo in animals with a retinal circulation similar to that of humans, and oximetry, which can be used non-invasively in both animals and humans to measure O2 concentration in retinal vessels. Microelectrodes uniquely have high spatial resolution, allowing the mapping of PO2 in detail, and when combined with mathematical models of diffusion and consumption, they provide information about retinal metabolism. Mathematical models, grounded in experiments, can also be used to simulate situations that are not amenable to experimental study. New methods of oximetry, particularly photoacoustic ophthalmoscopy and visible light optical coherence tomography, provide depth-resolved methods that can separate signals from blood vessels and surrounding tissues, and can be combined with blood flow measures to determine metabolic rate. We discuss the effects on retinal oxygenation of illumination, hypoxia and hyperoxia, and describe retinal oxygenation in diabetes, retinal detachment, arterial occlusion, and macular degeneration. We explain how the metabolic measurements obtained from microelectrodes and imaging are different, and how they need to be brought together in the future. Finally, we argue for revisiting the clinical use of hyperoxia in ophthalmology, particularly in retinal arterial occlusions and retinal detachment, based on animal research and diffusion theory.
Keywords: Animal; Diabetes; Human; Hyperoxia; Hypoxia; Macular degeneration; Oximetry; Oxygen; Oxygen microelectrode; Retina; Retinal detachment; Retinal metabolism.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


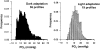



References
-
- Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion – what is currently thought? Acta Physiol (Oxf) 2011;202:253–269. - PubMed
-
- Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. - PubMed
-
- Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–118. - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

