Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress
- PMID: 28110218
- PMCID: PMC5256672
- DOI: 10.1016/j.redox.2016.12.035
Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress
Abstract
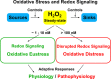
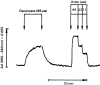
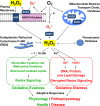
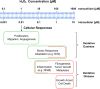
Hydrogen peroxide emerged as major redox metabolite operative in redox sensing, signaling and redox regulation. Generation, transport and capture of H2O2 in biological settings as well as their biological consequences can now be addressed. The present overview focuses on recent progress on metabolic sources and sinks of H2O2 and on the role of H2O2 in redox signaling under physiological conditions (1-10nM), denoted as oxidative eustress. Higher concentrations lead to adaptive stress responses via master switches such as Nrf2/Keap1 or NF-κB. Supraphysiological concentrations of H2O2 (>100nM) lead to damage of biomolecules, denoted as oxidative distress. Three questions are addressed: How can H2O2 be assayed in the biological setting? What are the metabolic sources and sinks of H2O2? What is the role of H2O2 in redox signaling and oxidative stress?
Keywords: H(2)O(2); Mitochondria; NADPH oxidases; Oxidative stress; Peroxiporins; Redox regulation.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

