Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies
- PMID: 28110394
- PMCID: PMC5603178
- DOI: 10.1007/s40265-017-0690-8
Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies
Abstract
Adoptive immunotherapy with chimeric antigen receptor-modified (CAR)-T cells is a rapidly growing therapeutic approach to treating patients with refractory cancer, with over 100 clinical trials in various malignancies in progress. The enthusiasm for CAR-T cells has been driven by the clinical success of CD19-targeted CAR-T cell therapy in B-cell acute lymphoblastic leukemia, and the promising data in B-cell non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Despite the success of targeting CD19 with CAR-T cells in early clinical studies, many challenges remain to improve outcomes, reduce toxicity, and determine the appropriate settings for CAR-T cell immunotherapy. Reviewing the lessons learned thus far in CD19 CAR-T cell trials and how some of these challenges may be overcome will help guide the development of CAR-T cell therapy for malignancies of B-cell origin, as well as for other hematopoietic and non-hematopoietic cancers.
Figures

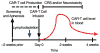
References
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. - PubMed
-
- Turtle CJ. Chimeric antigen receptor modified T cell therapy for B cell malignancies. Int J Hematol. 2014;99:132–140. - PubMed
-
- Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

