Defining the Ideal Time Interval Between Planned Induction Therapy and Surgery for Stage IIIA Non-Small Cell Lung Cancer
- PMID: 28110809
- PMCID: PMC5444908
- DOI: 10.1016/j.athoracsur.2016.09.053
Defining the Ideal Time Interval Between Planned Induction Therapy and Surgery for Stage IIIA Non-Small Cell Lung Cancer
Abstract
Background: Induction therapy leads to significant improvement in survival for selected patients with stage IIIA non-small cell lung cancer. The ideal time interval between induction therapy and surgery remains unknown.
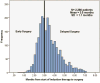
Methods: Clinical stage IIIA non-small cell lung cancer patients receiving induction therapy and surgery were identified in the National Cancer Database. Delayed surgery was defined as greater than or equal to 3 months after starting induction therapy. A logistic regression model identified variables associated with delayed surgery. Cox proportional hazards modeling and Kaplan-Meier analysis were performed to evaluate variables independently associated with overall survival.
Results: From 2006 to 2010, 1,529 of 2,380 (64.2%) received delayed surgery. Delayed surgery patients were older (61.2 ± 10.0 years versus 60.3 ± 9.2; p = 0.03), more likely to be non-white (12.4% versus 9.7%; p = 0.046), and less likely to have private insurance (50% versus 58.2%; p = 0.002). Delayed surgery patients were also more likely to have a sublobar resection (6.3% versus 2.9%). On multivariate analysis, age greater than 68 years (odds ratio [OR], 1.37; 95% confidence interval [CI], 1.1 to 1.7) was associated with delayed surgery, whereas white race (OR, 0.75; 95% CI, 0.57 to 0.99) and private insurance status (OR, 0.82; 95% CI, 0.68 to 0.99) were associated with early surgery. Delayed surgery was associated with higher risk of long-term mortality (hazard ratio, 1.25; 95% CI, 1.07 to 1.47).
Conclusions: Delayed surgery after induction therapy for stage IIIA lung cancer is associated with shorter survival, and is influenced by both social and physiologic factors. Prospective work is needed to further characterize the relationship between patient comorbidities and functional status with receipt of timely surgery.
Copyright © 2017 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Surveillance, Epidemiology, and End Results Program. SEER Statistical Fact Sheets: Lung and Bronchus Cancer. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed 1/23/2016.
-
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. European Organization for Research and Treatments of Cancer-Lung Cancer Group Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 2.2016. http://www.NCCN.org. Accessed 1/23/2016. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

