Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia
- PMID: 28111081
- PMCID: PMC5600182
- DOI: 10.1016/j.neuron.2016.12.021
Phosphatidylserine Exposure Controls Viral Innate Immune Responses by Microglia
Abstract
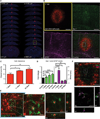
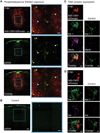
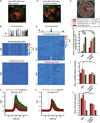
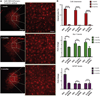
Microglia are the intrinsic immune sentinels of the central nervous system. Their activation restricts tissue injury and pathogen spread, but in some settings, including viral infection, this response can contribute to cell death and disease. Identifying mechanisms that control microglial responses is therefore an important objective. Using replication-incompetent adenovirus 5 (Ad5)-based vectors as a model, we investigated the mechanisms through which microglia recognize and respond to viral uptake. Transgenic, immunohistochemical, molecular-genetic, and fluorescence imaging approaches revealed that phosphatidylserine (PtdSer) exposure on the outer leaflet of transduced cells triggers their engulfment by microglia through TAM receptor-dependent mechanisms. We show that inhibition of phospholipid scramblase 1 (PLSCR1) activity reduces intracellular calcium dysregulation, prevents PtdSer externalization, and enables months-long protection of vector-transduced, transgene-expressing cells from microglial phagocytosis. Our study identifies PLSCR1 as a potent target through which the innate immune response to viral vectors, and potentially other stimuli, may be controlled.
Keywords: TAM receptor; adenovirus; astrocytes; calcium; infection; microglia; phagocytosis; phosphatidylserine; phospholipid scramblase 1; two-photon imaging.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Amengual O, Atsumi T, Oku K, Suzuki E, Horita T, Yasuda S, Koike T. Phospholipid scramblase 1 expression is enhanced in patients with antiphospholipid syndrome. Mod. Rheumatol. 2013;23:81–88. - PubMed
-
- Ben-Efraim I, Zhou Q, Wiedmer T, Gerace L, Sims PJ. Phospholipid scramblase 1 is imported into the nucleus by a receptor-mediated pathway and interacts with DNA. Biochemistry. 2004;43:3518–3526. - PubMed
-
- Bernales I, Fullaondo A, Marín-Vidalled MJ, Ucar E, Martínez-Taboada V, López-Hoyos M, Zubiaga AM. Innate immune response gene expression profiles characterize primary antiphospholipid syndrome. Genes Immun. 2008;9:38–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

