GLUT4 Mobilization Supports Energetic Demands of Active Synapses
- PMID: 28111082
- PMCID: PMC5330257
- DOI: 10.1016/j.neuron.2016.12.020
GLUT4 Mobilization Supports Energetic Demands of Active Synapses
Abstract
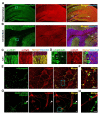
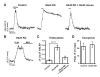
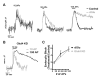
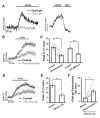
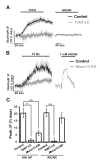
The brain is highly sensitive to proper fuel availability as evidenced by the rapid decline in neuronal function during ischemic attacks and acute severe hypoglycemia. We previously showed that sustained presynaptic function requires activity-driven glycolysis. Here, we provide strong evidence that during action potential (AP) firing, nerve terminals rely on the glucose transporter GLUT4 as a glycolytic regulatory system to meet the activity-driven increase in energy demands. Activity at synapses triggers insertion of GLUT4 into the axonal plasma membrane driven by activation of the metabolic sensor AMP kinase. Furthermore, we show that genetic ablation of GLUT4 leads to an arrest of synaptic vesicle recycling during sustained AP firing, similar to what is observed during acute glucose deprivation. The reliance on this biochemical regulatory system for "exercising" synapses is reminiscent of that occurring in exercising muscle to sustain cellular function and identifies nerve terminals as critical sites of proper metabolic control.
Keywords: GLUT4; glucose transport; glycolysis; neuronal metabolism; presynaptic function; vesicle cycle.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

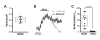

References
-
- Burchfield JG, Lu J, Fazakerley DJ, Tan SX, Ng Y, Mele K, Buckley MJ, Han W, Hughes WE, James DE. Novel systems for dynamically assessing insulin action in live cells reveals heterogeneity in the insulin response. Traffic. 2013;14:259–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

