Long-term evaluation of mucosal and systemic immunity and protection conferred by different polio booster vaccines
- PMID: 28111147
- PMCID: PMC5517362
- DOI: 10.1016/j.vaccine.2016.12.061
Long-term evaluation of mucosal and systemic immunity and protection conferred by different polio booster vaccines
Abstract
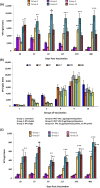
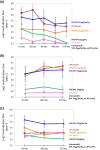
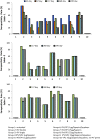
Oral polio vaccine (OPV) and Inactivated Polio Vaccine (IPV) have distinct advantages and limitations. IPV does not provide mucosal immunity and introduction of IPV to mitigate consequences of circulating vaccine-derived polio virus from OPV has very limited effect on transmission and OPV campaigns are essential for interrupting wild polio virus transmission, even in developed countries with a high coverage of IPV and protected sewer systems. The problem is magnified in many countries with limited resources. Requirement of refrigeration for storage and transportation for both IPV and OPV is also a major challenge in developing countries. Therefore, we present here long-term studies on comparison of a plant-based booster vaccine, which is free of virus and cold chain with IPV boosters and provide data on mucosal and systemic immunity and protection conferred by neutralizing antibodies. Mice were primed subcutaneously with IPV and boosted orally with lyophilized plant cells containing 1μg or 25μg polio viral protein 1 (VP1), once a month for three months or a single booster one year after the first prime. Our results show that VP1-IgG1 titers in single or double dose IPV dropped to background levels after one year of immunization. This decrease correlated with >50% reduction in seropositivity in double dose and <10% seropositivity in single dose IPV against serotype 1. Single dose IPV offered no or minimal protection against serotype 1 and 2 but conferred protection against serotype 3. VP1-IgA titers were negligible in IPV single or double dose vaccinated mice. VP1 antigen with two plant-derived adjuvants induced significantly high level and long lasting VP1-IgG1, IgA and neutralizing antibody titers (average 4.3-6.8 log2 titers). Plant boosters with VP1 and plant derived adjuvants maintained the same level titers from 29 to 400days and conferred the same level of protection against all three serotypes throughout the duration of this study. Even during period, when no plant booster was given (∼260days), VP1-IgG1 titers were maintained at high levels. Lyophilized plant cells expressing VP1 can be stored without losing efficacy, eliminating cold chain. Virus-free, cold-chain free vaccine is ready for further clinical development.
Keywords: Bioencapsulated plant cells; Oral delivery; Polio viral protein 1 (VP1); Poliovirus.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Mueller S., Wimmer E., Cello J. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res. 2005;111(2):175–193. - PubMed
-
- Shulman L.M., Gavrilin E., Jorba J. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel. Euro Surveill. 2014;19(7):20709. - PubMed
-
- Yang W., Terasaki T., Shiroki K. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229(2):421–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

