Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction
- PMID: 28111350
- PMCID: PMC5403568
- DOI: 10.1016/j.hrthm.2017.01.027
Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction
Abstract
Background: Interleukin 1β (IL-1β) is a key regulator of the inflammatory response after myocardial infarction (MI) by modulating immune cell recruitment, cytokine production, and extracellular matrix turnover. Elevated levels of IL-1β are associated with adverse remodeling, and inhibition of IL-1 signaling after MI results in improved contractile function.
Objective: The goal of this study was to determine whether IL-1 signaling also contributes to post-MI arrhythmogenesis.
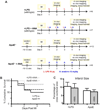
Methods: MI was created in 2 murine models of elevated inflammation: atherosclerotic on the Western diet or wild-type with a subseptic dose of lipopolysaccharide. The role of IL-1β was assessed with the IL-1 receptor antagonist anakinra (10 mg/(kg·d), starting 24 hours post-MI).
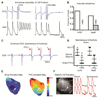
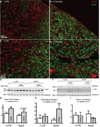
Results: In vivo and ex vivo molecular imaging showed reduced myocardial inflammation after a 4-day course of anakinra treatment, despite no change in infarct size. At day 5 post-MI, high-speed optical mapping of transmembrane potential and intracellular Ca2+ in isolated hearts revealed that IL-1β inhibition improved conduction velocity, reduced action potential duration dispersion, improved intracellular Ca2+ handling, decreased transmembrane potential and Ca2+ alternans magnitude, and reduced spontaneous and inducible ventricular arrhythmias. These functional improvements were linked to increased expression of connexin 43 and sarcoplasmic reticulum Ca2+-ATPase.
Conclusion: This study revealed a novel mechanism for IL-1β in contributing to defective excitation-contraction coupling and arrhythmogenesis in the post-MI heart. Our results suggest that inhibition of IL-1 signaling post-MI may represent a novel antiarrhythmic therapy.
Keywords: Action potentials; Antiarrhythmic agents; Calcium; Interleukins; Myocardial infarction.
Copyright © 2017 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Interleukin 1 receptor inhibition dampens the flame of postinfarction arrhythmias.Heart Rhythm. 2017 May;14(5):737-738. doi: 10.1016/j.hrthm.2017.02.018. Epub 2017 Feb 20. Heart Rhythm. 2017. PMID: 28223191 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

