Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia
- PMID: 28111464
- PMCID: PMC5555835
- DOI: 10.1038/leu.2017.32
Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia
Abstract
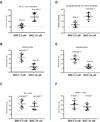
Although the BTK inhibitor ibrutinib has transformed the management of patients with chronic lymphocytic leukemia (CLL), it does not induce substantial apoptosis in vitro, and as such the mechanisms underlying its ability to kill CLL cells are not well understood. Acalabrutinib, a more specific BTK inhibitor now in development, also appears to be highly effective in CLL, but the connection of its mechanism with CLL cell death is also unclear. Using dynamic BH3 profiling, we analyzed alterations in the function of the mitochondrial apoptotic pathway induced by ibrutinib and acalabrutinib. We studied CLL patient samples treated ex vivo with both drugs, as well as primary samples from CLL patients on clinical trials of both drugs. We found that BTK inhibition enhances mitochondrial BCL-2 dependence without significantly altering overall mitochondrial priming. Enhancement of BCL-2 dependence was accompanied by an increase in the pro-apoptotic protein BIM. In contrast, treatment with the selective BCL-2 inhibitor venetoclax enhanced overall mitochondrial priming without increasing BCL-2 dependence. Pre-treatment of CLL cells with either BTK inhibitor, whether ex vivo or in vivo in patients, enhanced killing by venetoclax. Our data suggest that BTK inhibition enhances mitochondrial BCL-2 dependence, supporting the ongoing development of clinical trials combining BTK and BCL-2 inhibition.
Conflict of interest statement
J.D., E.I, and S.F. have no relevant conflicts of interest.
Figures






References
-
- Wiestner A. BCR pathway inhibition as therapy for chronic lymphocytic leukemia and lymphoplasmacytic lymphoma. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2014 Dec 5;2014(1):125–134. - PubMed
-
- Burger JA, Styles L, Kipps TJ. Ibrutinib for Chronic Lymphocytic Leukemia. N Engl J Med. 2016 Apr 21;374(16):1594–1595. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

