Divergent Synthesis of Quinolone Natural Products from Pseudonocardia sp. CL38489
- PMID: 28111524
- PMCID: PMC5215369
- DOI: 10.1002/ejoc.201601195
Divergent Synthesis of Quinolone Natural Products from Pseudonocardia sp. CL38489
Abstract
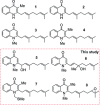
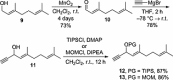
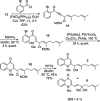
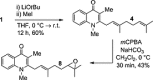
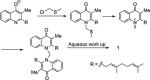
Two divergent synthetic routes are reported offering access to four quinolone natural products from Pseudonocardia sp. CL38489. Key steps to the natural products involved a regioselective epoxidation, an intramolecular Buchwald-Hartwig amination and a final acid-catalysed 1,3-allylic-alcohol rearrangement to give two of the natural products in one step. This study completes the synthesis of all eight antibacterial quinolone natural products reported in the family. In addition, this modular strategy enables an improved synthesis towards two natural products previously reported.
Keywords: Antibacterial agents; Cross‐coupling; Michael addition; Natural products; Quinolones.
Figures
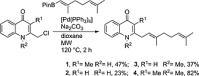


References
-
- Van Bambeke F., Michot J.‐M., Van Eldere J. and Tulkens P. M., Clin. Microbiol. Infect., 2005, 11, 256–280. - PubMed
-
- Hays E. E. and Wells I. C., J. Biol. Chem., 1945, 159, 725–750.
-
- Machan Z. A., Taylor G. W., Pitt T. L., Cole P. J. and Wilson R., J. Antimicrob. Chemother., 1992, 30, 615–623. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
