Japanese Classification of Esophageal Cancer, 11th Edition: part II and III
- PMID: 28111536
- PMCID: PMC5222925
- DOI: 10.1007/s10388-016-0556-2
Japanese Classification of Esophageal Cancer, 11th Edition: part II and III
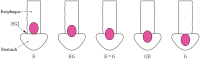
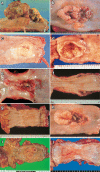
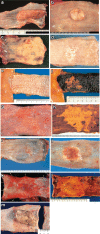
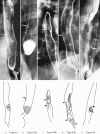
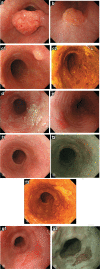
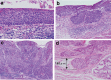
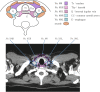
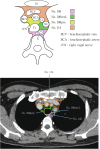
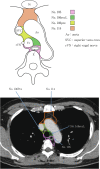
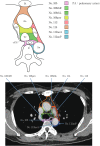
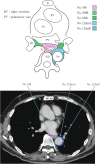
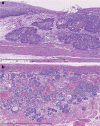
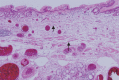
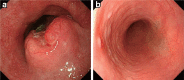
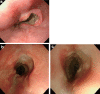
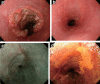
Figures




























LinkOut - more resources
Full Text Sources
Other Literature Sources
