Dynamic Expression of Serotonin Receptor 5-HT3A in Developing Sensory Innervation of the Lower Urinary Tract
- PMID: 28111539
- PMCID: PMC5216032
- DOI: 10.3389/fnins.2016.00592
Dynamic Expression of Serotonin Receptor 5-HT3A in Developing Sensory Innervation of the Lower Urinary Tract
Abstract
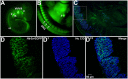
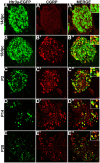
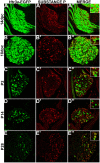
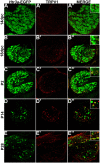
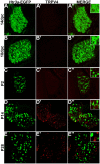
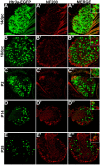
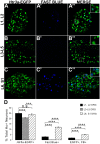
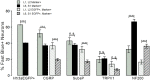
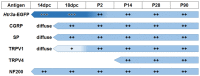
Sensory afferent signaling is required for normal function of the lower urinary tract (LUT). Despite the wide prevalence of bladder dysfunction and pelvic pain syndromes, few effective treatment options are available. Serotonin receptor 5-HT3A is a known mediator of visceral afferent signaling and has been implicated in bladder function. However, basic expression patterns for this gene and others among developing bladder sensory afferents that could be used to inform regenerative efforts aimed at treating deficiencies in pelvic innervation are lacking. To gain greater insight into the molecular characteristics of bladder sensory innervation, we conducted a thorough characterization of Htr3a expression in developing and adult bladder-projecting lumbosacral dorsal root ganglia (DRG) neurons. Using a transgenic Htr3a-EGFP reporter mouse line, we identified 5-HT3A expression at 10 days post coitus (dpc) in neural crest derivatives and in 12 dpc lumbosacral DRG. Using immunohistochemical co-localization we observed Htr3a-EGFP expression in developing lumbosacral DRG that partially coincides with neuropeptides CGRP and Substance P and capsaicin receptor TRPV1. A majority of Htr3a-EGFP+ DRG neurons also express a marker of myelinated Aδ neurons, NF200. There was no co-localization of 5-HT3A with the TRPV4 receptor. We employed retrograde tracing in adult Htr3a-EGFP mice to quantify the contribution of 5-HT3A+ DRG neurons to bladder afferent innervation. We found that 5-HT3A is expressed in a substantial proportion of retrograde traced DRG neurons in both rostral (L1, L2) and caudal (L6, S1) axial levels that supply bladder innervation. Most bladder-projecting Htr3a-EGFP+ neurons that co-express CGRP, Substance P, or TRPV1 are found in L1, L2 DRG, whereas Htr3a-EGFP+, NF200+ bladder-projecting neurons are from the L6, S1 axial levels. Our findings contribute much needed information regarding the development of LUT innervation and highlight the 5-HT3A serotonin receptor as a candidate for future studies of neurally mediated bladder control.
Keywords: autonomic nervous system; dorsal root ganglia; lower urinary tract; neural crest; serotonin.
Figures
References
-
- Baiou D., Santha P., Avelino A., Charrua A., Bacskai T., Matesz K., et al. (2007). Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J. Comp. Neurol. 503, 334–347. 10.1002/cne.21389 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

