Genome Editing of Wnt-1, a Gene Associated with Segmentation, via CRISPR/Cas9 in the Pine Caterpillar Moth, Dendrolimus punctatus
- PMID: 28111552
- PMCID: PMC5216022
- DOI: 10.3389/fphys.2016.00666
Genome Editing of Wnt-1, a Gene Associated with Segmentation, via CRISPR/Cas9 in the Pine Caterpillar Moth, Dendrolimus punctatus
Abstract
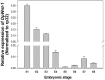
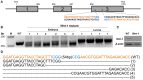
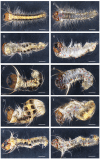
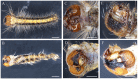
The pine caterpillar moth, Dendrolimus punctatus, is a devastating forest pest. Genetic manipulation of this insect pest is limited due to the lack of genomic and functional genomic toolsets. Recently, CRISPR/Cas9 technology has been demonstrated to be a promising approach to modify the genome. To investigate gene functions during the embryogenesis, we introduced CRISPR/Cas9 system in D. punctatus to precisely and effectively manipulate gene expressions inmutant embryos. Compared to controls, knocking out of DpWnt-1, a gene well known for its role in the early body planning, led to high embryonic mortality. Among these mutants, 32.9% of the embryos and larvae showed an abnormal development. DpWnt-1 mutants predominantly exhibited abnormal posterior segments. In addition, multiple phenotypes were observed, including the loss of limbs and the head deformation, suggesting that DpWnt-1 signaling pathway is necessary for anterior segmentation and appendage development. Overall, our results demonstrate that CRISPR/Cas9 system is feasible and efficient in inducing mutations at a specific locus in D. punctatus. This study not only lays the foundation for characterizing gene functions in a non-model species, but also facilitates the future development of pest control alternatives for a major defoliator.
Keywords: CRISPR/Cas9; Dendrolimus punctatus; Wnt-1; embryogenesis; genome editing; segmentation.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

