Benzyl isothiocyanate induces protective autophagy in human lung cancer cells through an endoplasmic reticulum stress-mediated mechanism
- PMID: 28112178
- PMCID: PMC5386313
- DOI: 10.1038/aps.2016.146
Benzyl isothiocyanate induces protective autophagy in human lung cancer cells through an endoplasmic reticulum stress-mediated mechanism
Abstract
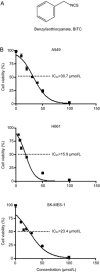
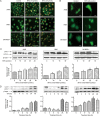
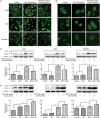
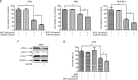
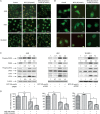
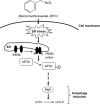
Isothiocyanates, such as allyl isothiocya¬nate (AITC), benzyl isothiocyanate (BITC), phenethyl isothio¬cyanate (PEITC) and sulforaphane (SFN), are natural compounds abundant in cruciferous vegetables, which have substantial chemopreventive activities against various human malignancies. However, the mechanisms underlying the inhibition of tumor cell growth by isothiocyanates are not fully understood. Since autophagy has dual functions in cancer, in the present study we investigated the effects of BITC on autophagy induction in human lung cancer cells in vitro and in vivo. BITC (1-100 μmol/L) dose-dependently inhibited the growth of 3 different human lung cancer cell lines A549 (adenocarcinoma), H661 (large cell carcinoma) and SK-MES-1 (squamous cell carcinoma) with IC50 values of 30.7±0.14, 15.9±0.22 and 23.4±0.11 μmol/L, respectively. BITC (10-40 μmol/L) induced autophagy in the lung cancer cells, evidenced by the formation of acidic vesicular organelles (AVOs), the accumulation of LC3-II, the punctate pattern of LC3, and the expression of Atg5. Pretreatment with the autophagy inhibitor 3-MA (5 mmol/L) significantly enhanced the BITC-caused growth inhibition in the lung cancer cells. Furthermore, BITC (20-40 μmol/L) activated ER stress, as shown by the increased cytosolic Ca2+ level and the phosphorylation of the ER stress marker proteins PERK and eIF2α in the lung cancer cells. Pretreatment with the ER stress inhibitor 4-PBA (5 mmol/L) attenuated the autophagy induction and potentiated the BITC-induced cell growth inhibition. In nude mice bearing A549 xenografts, administration of BITC (100 mg·kg-1·d-1, ip) for 8 weeks markedly suppressed the lung tumor growth, and significantly enhanced both autophagy and ER stress in the tumor tissues. Our results demonstrate that BITC inhibits human lung cancer cell growth in vitro and in vivo. In addition, BITC induces autophagy in the lung cancer cells, which protects the cancer cells against the inhibitory action of BITC; the autophagy induction is mediated by the ER stress response.
Figures


References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. - PubMed
-
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–71. - PubMed
-
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011; 147: 728–41. - PubMed
-
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013; 368: 1845–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

