A human immunodeficiency syndrome caused by mutations in CARMIL2
- PMID: 28112205
- PMCID: PMC5473639
- DOI: 10.1038/ncomms14209
A human immunodeficiency syndrome caused by mutations in CARMIL2
Abstract
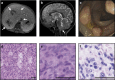
Human T-cell function is dependent on T-cell antigen receptor (TCR) and co-signalling as evidenced by immunodeficiencies affecting TCR-dependent signalling pathways. Here, we show four human patients with EBV+ disseminated smooth muscle tumours that carry two homozygous loss-of-function mutations in the CARMIL2 (RLTPR) gene encoding the capping protein regulator and myosin 1 linker 2. These patients lack regulatory T cells without evidence of organ-specific autoimmunity, and have defective CD28 co-signalling associated with impaired T-cell activation, differentiation and function, as well as perturbed cytoskeletal organization associated with T-cell polarity and migration disorders. Human CARMIL2-deficiency is therefore an autosomal recessive primary immunodeficiency disorder associated with defective CD28-mediated TCR co-signalling and impaired cytoskeletal dynamics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Milner J. D. & Holland S. M. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat. Rev. Immunol. 13, 635–648 (2013). - PubMed
-
- Fischer A. Recent advances in understanding the pathophysiology of primary T cell immunodeficiencies. Trends. Mol. Med. 21, 408–416 (2015). - PubMed
-
- Veillette A., Perez-Quintero L. A. & Latour S. X-linked lymphoproliferative syndromes and related autosomal recessive disorders. Curr. Opin. Allergy Clin. Immunol. 13, 614–622 (2013). - PubMed
-
- Wilson E. H. & Hunter C. A. Understanding the role of the CD40–CD40L interaction in resistance to parasitic infections. Parasite Immunol. 25, 179–183 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

