Protein kinase C in cancer: The top five unanswered questions
- PMID: 28112438
- PMCID: PMC5423816
- DOI: 10.1002/mc.22617
Protein kinase C in cancer: The top five unanswered questions
Abstract
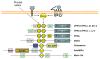
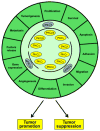
Few kinases have been studied as extensively as protein kinase C (PKC), particularly in the context of cancer. As major cellular targets for the phorbol ester tumor promoters and diacylglycerol (DAG), a second messenger generated by stimulation of membrane receptors, PKC isozymes play major roles in the control of signaling pathways associated with proliferation, migration, invasion, tumorigenesis, and metastasis. However, despite decades of research, fundamental questions remain to be answered or are the subject of intense controversy. Primary among these unresolved issues are the role of PKC isozymes as either tumor promoter or tumor suppressor kinases and the incomplete understanding on isozyme-specific substrates and effectors. The involvement of PKC isozymes in cancer progression needs to be reassessed in the context of specific oncogenic and tumor suppressing alterations. In addition, there are still major hurdles in addressing isozyme-specific function due to the limited specificity of most pharmacological PKC modulators and the lack of validated predictive biomarkers for response, which impacts the translation of these agents to the clinic. In this review we focus on key controversial issues and upcoming challenges, with the expectation that understanding the intricacies of PKC function will help fulfill the yet unsuccessful promise of targeting PKCs for cancer therapeutics.
Keywords: cancer therapy; oncogene; phorbol esters; protein kinase C; tumor promoter; tumor suppressor.
© 2017 Wiley Periodicals, Inc.
Figures
References
-
- Castagna M, Takai Y, Kaibuchi K, et al. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. - PubMed
-
- Konig B, Di Nitto PA, Blumberg PM. Phospholipid and Ca++ dependency of phorbol ester receptors. J Cell Biochem. 1985;27:255–265. - PubMed
-
- Takai Y, Kishimoto A, Iwasa Y, et al. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254:3692–3695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

