Radiology Reports With Hyperlinks Improve Target Lesion Selection and Measurement Concordance in Cancer Trials
- PMID: 28112557
- PMCID: PMC6762032
- DOI: 10.2214/AJR.16.16845
Radiology Reports With Hyperlinks Improve Target Lesion Selection and Measurement Concordance in Cancer Trials
Abstract
Objective: Radiology reports often lack the measurements of target lesions that are needed for oncology clinical trials. When available, the measurements in the radiology reports often do not match those in the records used to calculate therapeutic response. This study assessed the clinical value of hyperlinked tumor measurements in multimedia-enhanced radiology reports in the PACS and the inclusion of a radiologist assistant in the process of assessing tumor burden.
Materials and methods: We assessed 489 target lesions in 232 CT examinations of 71 patients with metastatic genitourinary cancer enrolled in two therapeutic trials. We analyzed target lesion selection and measurement concordance between oncology records (used to calculate therapeutic response) and two types of radiology reports in the PACS: multimedia-enhanced radiology reports and text-only reports. For statistical tests, we used the Wilcoxon signed rank, Wilcoxon rank sum test, and Fisher method to combine p values from the paired and unpaired results. The Fisher exact test was used to compare overall measurement concordance.
Results: Concordance on target lesion selection was greater for multimedia-enhanced radiology reports (78%) than the text-only reports (52%) (p = 0.0050). There was also improved overall measurement concordance with the multimedia-enhanced radiology reports (68%) compared with the text-only reports (38%) (p < 0.0001).
Conclusion: Compared with text-only reports, hyperlinked multimedia-enhanced radiology reports improved concordance of target lesion selection and measurement with the measurements used to calculate therapeutic response.
Keywords: Response Evaluation Criteria in Solid Tumors (RECIST) 1.1; hyperlinks; multimedia; radiology reports; tumor assessment.
Figures

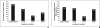



References
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
-
- Chalian H, Tore HG, Horowitz JM, et al. Radiologic assessment of response to therapy: comparison of RECIST versions 1.1 and 1.0. RadioGraphics. 2011;31:2093–2105. - PubMed
-
- Sevenster M, Travis AR, Ganesh RK, et al. Improved efficiency in clinical workflow of reporting measured oncology lesions via PACS-integrated lesion tracking tool. AJR. 2015;204:576–583. - PubMed
-
- Kahn CE, Langlotz CP, Burnside ES, et al. Toward best practices in radiology reporting. Radiology. 2009;252:852–856. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

