Structural basis of dual Ca2+/pH regulation of the endolysosomal TRPML1 channel
- PMID: 28112729
- PMCID: PMC5336481
- DOI: 10.1038/nsmb.3362
Structural basis of dual Ca2+/pH regulation of the endolysosomal TRPML1 channel
Abstract
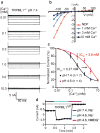
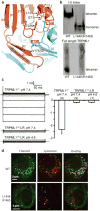
The activities of organellar ion channels are often regulated by Ca2+ and H+, which are present in high concentrations in many organelles. Here we report a structural element critical for dual Ca2+/pH regulation of TRPML1, a Ca2+-release channel crucial for endolysosomal function. TRPML1 mutations cause mucolipidosis type IV (MLIV), a severe lysosomal storage disorder characterized by neurodegeneration, mental retardation and blindness. We obtained crystal structures of the 213-residue luminal domain of human TRPML1 containing three missense MLIV-causing mutations. This domain forms a tetramer with a highly electronegative central pore formed by a novel luminal pore loop. Cysteine cross-linking and cryo-EM analyses confirmed that this architecture occurs in the full-length channel. Structure-function studies demonstrated that Ca2+ and H+ interact with the luminal pore and exert physiologically important regulation. The MLIV-causing mutations disrupt the luminal-domain structure and cause TRPML1 mislocalization. Our study reveals the structural underpinnings of TRPML1's regulation, assembly and pathogenesis.
Figures
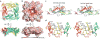
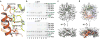


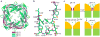
References
-
- Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

