Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs
- PMID: 28114170
- PMCID: PMC7228580
- DOI: 10.1097/TP.0000000000001646
Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs
Erratum in
-
Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs: Erratum.Transplantation. 2018 Feb;102(2):e88. doi: 10.1097/TP.0000000000002066. Transplantation. 2018. PMID: 29381648 Free PMC article. No abstract available.
Abstract
Background: Antipig antibodies are a barrier to clinical xenotransplantation. We evaluated antibody binding of waitlisted renal transplant patients to 3 glycan knockout (KO) pig cells and class I swine leukocyte antigens (SLA).
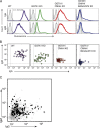
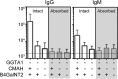
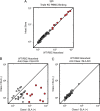
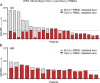
Methods: Peripheral blood mononuclear cells from SLA identical wild type (WT), α1, 3-galactosyltransferase (GGTA1) KO, GGTA1/ cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) KO, and GGTA1/ CMAH /b1,4 N-acetylgalactosaminyl transferase (B4GalNT2) KO pigs were screened for human antibody binding using flow cytometric crossmatch (FCXM). Sera from 820 patients were screened on GGTA1/CMAH/B4GalNT2 KO cells and a subset with elevated binding was evaluated further. FCXM was performed on SLA intact cells and GGTA1/SLA class I KO cells after depletion with WT pig RBCs to remove cell surface reactive antibodies, but leave SLA antibodies. Lastly, human and pig reactive antibodies were eluted and tested for cross-species binding and reactivity to single-antigen HLA beads.
Results: Sequential glycan KO modifications significantly reduce antibody binding of waitlisted patients. Sera exhibiting elevated binding without reduction after depletion with WT RBCs demonstrate reduced binding to SLA class I KO cells. Human IgG, eluted from human and pig peripheral blood mononuclear cells, interacted across species and bound single-antigen HLA beads in common epitope-restricted patterns.
Conclusions: Many waitlisted patients have minimal xenoreactive antibody binding to the triple KO pig, but some HLA antibodies in sensitized patients cross-react with class I SLA. SLA class I is a target for genome editing in xenotransplantation.
Figures

Comment in
-
Reducing the Threshold for Clinical Renal Xenotransplantation.Transplantation. 2017 Apr;101(4):692-693. doi: 10.1097/TP.0000000000001641. Transplantation. 2017. PMID: 28099406 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

