Enzyme-catalyzed cationic epoxide rearrangements in quinolone alkaloid biosynthesis
- PMID: 28114276
- PMCID: PMC5310975
- DOI: 10.1038/nchembio.2283
Enzyme-catalyzed cationic epoxide rearrangements in quinolone alkaloid biosynthesis
Abstract
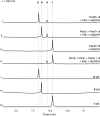
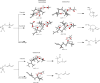
Epoxides are highly useful synthons and biosynthons for the construction of complex natural products during total synthesis and biosynthesis, respectively. Among enzyme-catalyzed epoxide transformations, a reaction that is notably missing, in regard to the synthetic toolbox, is cationic rearrangement that takes place under strong acid. This is a challenging transformation for enzyme catalysis, as stabilization of the carbocation intermediate upon epoxide cleavage is required. Here, we discovered two Brønsted acid enzymes that can catalyze two unprecedented epoxide transformations in biology. PenF from the penigequinolone pathway catalyzes a cationic epoxide rearrangement under physiological conditions to generate a quaternary carbon center, while AsqO from the aspoquinolone pathway catalyzes a 3-exo-tet cyclization to forge a cyclopropane-tetrahydrofuran ring system. The discovery of these new epoxide-modifying enzymes further highlights the versatility of epoxides in complexity generation during natural product biosynthesis.
Figures
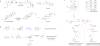


References
-
- Sienel G, Rieth R, Rowbottom KT. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. Epoxides.
-
- Jacobsen EN. Asymmetric catalysis of epoxide ring-opening reactions. Acc. Chem. Res. 2000;33:421–431. - PubMed
-
- Krake SH, Bergmeier SC. Inter- and intramolecular reactions of epoxides and aziridines with pi-nucleophiles. Tetrahedron. 2010;66:7337–7360.
-
- Pineschi M. Asymmetric ring-opening of epoxides and aziridines with carbon nucleophiles. Eur. J. Org. Chem. 2006;22:4979–4988.
ONLINE METHONDS REFERENCES
-
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Wallingford, CT, USA: 2009.
-
- Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241.
-
- Tomasi J, Mennucci B, Cammi R. Quantum mechanical continuum solvation models. Chem. Rev. 2005;105:2999–3093. - PubMed
-
- Simon L, Goodman JM. How reliable are DFT transition structures? Comparison of GGA, hybrid-meta-GGA and meta-GGA functionals. Org. Biomol. Chem. 2011;9:689–700. - PubMed
-
- Legault C. Université de Sherbrooke, Sherbrooke, Quebec, Canada. 2009
MeSH terms
Substances
Associated data
- PubChem-Substance/321903796
- PubChem-Substance/321903800
- PubChem-Substance/321903801
- PubChem-Substance/321903802
- PubChem-Substance/321903803
- PubChem-Substance/321903804
- PubChem-Substance/321903805
- PubChem-Substance/321903806
- PubChem-Substance/321903807
- PubChem-Substance/321903797
- PubChem-Substance/321903798
- PubChem-Substance/321903799
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

