Modelling Anti-Ov16 IgG4 Antibody Prevalence as an Indicator for Evaluation and Decision Making in Onchocerciasis Elimination Programmes
- PMID: 28114304
- PMCID: PMC5289624
- DOI: 10.1371/journal.pntd.0005314
Modelling Anti-Ov16 IgG4 Antibody Prevalence as an Indicator for Evaluation and Decision Making in Onchocerciasis Elimination Programmes
Abstract
Background: Onchocerciasis is targeted for elimination in Africa through annual or biannual ivermectin mass drug administration (MDA). An immunodiagnostic test, based on the detection of human IgG4 antibodies in the blood to the Onchocerca volvulus-specific antigen Ov16, is one of the recommended tools for determining whether transmission is interrupted and mass treatment can stop. For different transmission settings, the relationship between post-MDA Ov16 antibody prevalence in children (measured 1 year after the last round of MDA) and the duration and coverage of MDA, the mf prevalence in the population, and the probability that onchocerciasis is eventually eliminated is explored through mathematical modelling.
Methodology: The ONCHOSIM model was extended with new output on the Ov16 antibody serostatus of individuals. Seroconversion was assumed to be triggered by the first worm establishing in the host, with seroconversion occurring either before maturation, after maturation or only after the start of mf production. We are mainly interested in seroconversion rates in children, and for now ignore the possibility of seroreversion to simplify the model.
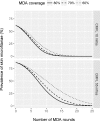
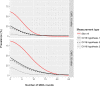
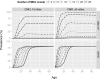
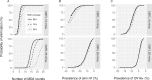
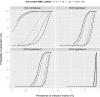
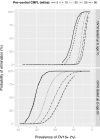
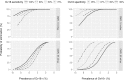
Principal findings: Yearly repeated MDA leads to a strong reduction in the parasite acquisition rate in humans. This reduces the seroconversion rate in newborns and young children, while those who seroconverted before the start of control remain antibody positive. Both the microfiladermia prevalence in the population aged 5 years and above and the Ov16 antibody prevalence in children under 10 declined with increasing duration of MDA. The association between either of these indicators and the model-predicted probability of elimination was not influenced much by the assumed treatment coverage levels, but was found to depend on baseline endemicity levels, assumptions regarding the trigger of seroconversion, and diagnostic test characteristics (sensitivity and specificity).
Conclusions: Better understanding of the dynamics of Ov16 antibody responses is required for accurate interpretation of seroprevalence data and more precise estimation of endpoint for MDA. Our study demonstrates that this endpoint will be dependent on baseline endemicity levels, which should be taken into account in guidelines for defining when to stop MDA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Analysis of age-dependent trends in Ov16 IgG4 seroprevalence to onchocerciasis.Parasit Vectors. 2016 Jun 13;9(1):338. doi: 10.1186/s13071-016-1623-1. Parasit Vectors. 2016. PMID: 27296630 Free PMC article.
-
Predictive Value of Ov16 Antibody Prevalence in Different Subpopulations for Elimination of African Onchocerciasis.Am J Epidemiol. 2019 Sep 1;188(9):1723-1732. doi: 10.1093/aje/kwz109. Am J Epidemiol. 2019. PMID: 31062838 Free PMC article.
-
Integrated seroprevalence-based assessment of Wuchereria bancrofti and Onchocerca volvulus in two lymphatic filariasis evaluation units of Mali with the SD Bioline Onchocerciasis/LF IgG4 Rapid Test.PLoS Negl Trop Dis. 2019 Jan 30;13(1):e0007064. doi: 10.1371/journal.pntd.0007064. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30699120 Free PMC article.
-
River Blindness: Mathematical Models for Control and Elimination.Adv Parasitol. 2016;94:247-341. doi: 10.1016/bs.apar.2016.08.003. Epub 2016 Oct 7. Adv Parasitol. 2016. PMID: 27756456 Review.
-
Assessment and monitoring of onchocerciasis in Latin America.Adv Parasitol. 2011;77:175-226. doi: 10.1016/B978-0-12-391429-3.00008-3. Adv Parasitol. 2011. PMID: 22137585 Review.
Cited by
-
Diagnostics for onchocerciasis in the era of elimination.Int Health. 2018 Mar 1;10(suppl_1):i20-i26. doi: 10.1093/inthealth/ihx047. Int Health. 2018. PMID: 29471336 Free PMC article. Review.
-
The World Health Organization 2030 goals for onchocerciasis: Insights and perspectives from mathematical modelling: NTD Modelling Consortium Onchocerciasis Group.Gates Open Res. 2019 Sep 26;3:1545. doi: 10.12688/gatesopenres.13067.1. eCollection 2019. Gates Open Res. 2019. PMID: 31723729 Free PMC article.
-
How Can Onchocerciasis Elimination in Africa Be Accelerated? Modeling the Impact of Increased Ivermectin Treatment Frequency and Complementary Vector Control.Clin Infect Dis. 2018 Jun 1;66(suppl_4):S267-S274. doi: 10.1093/cid/cix1137. Clin Infect Dis. 2018. PMID: 29860291 Free PMC article.
-
Assessment of multiplex Onchocerca volvulus peptide ELISA in non-endemic tropical regions.Parasit Vectors. 2019 Nov 29;12(1):570. doi: 10.1186/s13071-019-3824-x. Parasit Vectors. 2019. PMID: 31783767 Free PMC article.
-
Onchocerca volvulus infection in Tihama region - west of Yemen: Continuing transmission in ivermectin-targeted endemic foci and unveiled endemicity in districts with previously unknown status.PLoS Negl Trop Dis. 2018 Mar 5;12(3):e0006329. doi: 10.1371/journal.pntd.0006329. eCollection 2018 Mar. PLoS Negl Trop Dis. 2018. PMID: 29505580 Free PMC article.
References
-
- Remme JHF, Feenstra P, Lever PR, Medici AC, Morel CM, Noma M et al. Tropical diseases targeted for elimination: chagas diseases, lymphatic filariasis, onchocerciasis and leprosy In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB et al., editors. Disease Control Priorities in Developing Countries. Second ed.: Oxford University Press, World Bank; 2006. p. 433–50. - PubMed
-
- WHO/APOC. Report of the consultative meetings on strategic options and alternative treatment strategies for accelerating onchocerciasis elimination in Africa.2015. Report No.: WHO/MG/15.20.
-
- Traore MO, Sarr MD, Badji A, Bissan Y, Diawara L, Doumbia K et al. Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Negl Trop Dis. 2012;6(9):e1825 10.1371/journal.pntd.0001825 - DOI - PMC - PubMed
-
- African Programme for Onchocerciasis Control (WHO/APOC). Conceptual and operational framework of onchocerciasis elimination with ivermectin treatment. Ouagadougou: African Programme for Onchocerciasis Control, World Health Organization. 2010. Report No.: WHO/APOC/MG/10.1. Available from:http://www.who.int/apoc/oncho_elimination_report_english.pdf. Accessed 20 September 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

