A Unique Egg Cortical Granule Localization Motif Is Required for Ovastacin Sequestration to Prevent Premature ZP2 Cleavage and Ensure Female Fertility in Mice
- PMID: 28114310
- PMCID: PMC5293279
- DOI: 10.1371/journal.pgen.1006580
A Unique Egg Cortical Granule Localization Motif Is Required for Ovastacin Sequestration to Prevent Premature ZP2 Cleavage and Ensure Female Fertility in Mice
Abstract
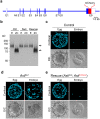
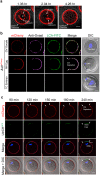
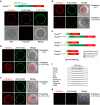
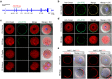
Monospermic fertilization is mediated by the extracellular zona pellucida composed of ZP1, ZP2 and ZP3. Sperm bind to the N-terminus of ZP2 which is cleaved after fertilization by ovastacin (encoded by Astl) exocytosed from egg cortical granules to prevent sperm binding. AstlNull mice lack the post-fertilization block to sperm binding and the ability to rescue this phenotype with AstlmCherry transgenic mice confirms the role of ovastacin in providing a definitive block to polyspermy. During oogenesis, endogenous ovastacin traffics through the endomembrane system prior to storage in peripherally located cortical granules. Deletion mutants of ovastacinmCherry expressed in growing oocytes define a unique 7 amino acid motif near its N-terminus that is necessary and sufficient for cortical granule localization. Deletion of the 7 amino acids by CRISPR/Cas9 at the endogenous locus (AstlΔ) prevents cortical granule localization of ovastacin. The misdirected enzyme is present within the endomembrane system and ZP2 is prematurely cleaved. Sperm bind poorly to the zona pellucida of AstlΔ/Δ mice with partially cleaved ZP2 and female mice are sub-fertile.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures
Similar articles
-
Intracellular activation of ovastacin mediates pre-fertilization hardening of the zona pellucida.Mol Hum Reprod. 2017 Sep 1;23(9):607-616. doi: 10.1093/molehr/gax040. Mol Hum Reprod. 2017. PMID: 28911209 Free PMC article.
-
Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy.J Cell Biol. 2012 Apr 2;197(1):37-44. doi: 10.1083/jcb.201112094. J Cell Biol. 2012. PMID: 22472438 Free PMC article.
-
Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy.Dev Cell. 2018 Sep 10;46(5):627-640.e5. doi: 10.1016/j.devcel.2018.07.020. Epub 2018 Aug 16. Dev Cell. 2018. PMID: 30122633 Free PMC article.
-
The mammalian egg's zona pellucida, fertilization, and fertility.Curr Top Dev Biol. 2025;162:207-258. doi: 10.1016/bs.ctdb.2024.10.008. Epub 2025 Feb 5. Curr Top Dev Biol. 2025. PMID: 40180510 Review.
-
The molecular basis of gamete recognition in mice and humans.Mol Hum Reprod. 2013 May;19(5):279-89. doi: 10.1093/molehr/gat004. Epub 2013 Jan 17. Mol Hum Reprod. 2013. PMID: 23335731 Free PMC article. Review.
Cited by
-
Dynein promotes porcine oocyte meiotic progression by maintaining cytoskeletal structures and cortical granule arrangement.Cell Cycle. 2017;16(21):2139-2145. doi: 10.1080/15384101.2017.1380133. Epub 2017 Oct 4. Cell Cycle. 2017. PMID: 28933593 Free PMC article.
-
Simulated microgravity reduces quality of ovarian follicles and oocytes by disrupting communications of follicle cells.NPJ Microgravity. 2023 Jan 23;9(1):7. doi: 10.1038/s41526-023-00248-5. NPJ Microgravity. 2023. PMID: 36690655 Free PMC article.
-
Crossing the barrier or how regulation of ovastacin controls fertilization and translates into clinical phenotypes.iScience. 2025 Jul 1;28(8):112976. doi: 10.1016/j.isci.2025.112976. eCollection 2025 Aug 15. iScience. 2025. PMID: 40697826 Free PMC article. Review.
-
Expression Analysis of ZPB2a and Its Regulatory Role in Sperm-Binding in Viviparous Teleost Black Rockfish.Int J Mol Sci. 2022 Aug 22;23(16):9498. doi: 10.3390/ijms23169498. Int J Mol Sci. 2022. PMID: 36012756 Free PMC article.
-
Intracellular activation of ovastacin mediates pre-fertilization hardening of the zona pellucida.Mol Hum Reprod. 2017 Sep 1;23(9):607-616. doi: 10.1093/molehr/gax040. Mol Hum Reprod. 2017. PMID: 28911209 Free PMC article.
References
-
- Stewart-Savage J, Bavister BD. A cell surface block to polyspermy occurs in golden hamster eggs. Dev Biol. 1988;128:150–7. - PubMed
-
- Maleszewski M, Kimura Y, Yanagimachi R. Sperm membrane incorporation into oolemma contributes to the oolemma block to sperm penetration: evidence based on intracytoplasmic sperm injection experiments in the mouse. Mol Reprod Dev. 1996;44(2):256–9. 10.1002/(SICI)1098-2795(199606)44:2<256::AID-MRD16>3.0.CO;2-0 - DOI - PubMed
-
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250(2):280–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

