A mechanistic kinetic description of lactate dehydrogenase elucidating cancer diagnosis and inhibitor evaluation
- PMID: 28114833
- PMCID: PMC6010104
- DOI: 10.1080/14756366.2016.1275606
A mechanistic kinetic description of lactate dehydrogenase elucidating cancer diagnosis and inhibitor evaluation
Abstract
As a key enzyme for glycolysis, lactate dehydrogenase (LDH) remains as a topic of great interest in cancer study. Though a number of kinetic models have been applied to describe the dynamic behavior of LDH, few can reflect its actual mechanism, making it difficult to explain the observed substrate and competitor inhibitions at wide concentration ranges. A novel mechanistic kinetic model is developed based on the enzymatic processes and the interactive properties of LDH. Better kinetic simulation as well as new enzyme interactivity information and kinetic properties extracted from published articles via the novel model was presented. Case studies were presented to a comprehensive understanding of the effect of temperature, substrate, and inhibitor on LDH kinetic activities for promising application in cancer diagnosis, inhibitor evaluation, and adequate drug dosage prediction.
Keywords: Cancer diagnosis; inhibitor evaluation; kinetic model; lactate dehydrogenase; oligomeric enzyme.
Figures



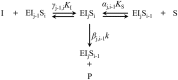



References
-
- O'Brien J, Kla KM, Hopkins IB, et al. . Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res 2007;32:597–607. - PubMed
-
- Nicholls D, Wood I, Nobbs T, et al. . Dissecting the contributions of a specific side-chain interaction to folding and catalysis of Bacillus stearothermophilus lactate dehydrogenase. Eur J Biochem 1993;212:447–55. - PubMed
-
- Robergs RA, Ghiasvand F, Parker D.. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol 2004;287:502–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
