TGF-β1-induced differentiation of SHED into functional smooth muscle cells
- PMID: 28114966
- PMCID: PMC5260045
- DOI: 10.1186/s13287-016-0459-0
TGF-β1-induced differentiation of SHED into functional smooth muscle cells
Abstract
Background: Adequate vascularization is crucial for supplying nutrition and discharging metabolic waste in freshly transplanted tissue-engineered constructs. Obtaining the appropriate building blocks for vascular tissue engineering (i.e. endothelial and mural cells) is a challenging task for tissue neovascularization. Hence, we investigated whether stem cells from human exfoliated deciduous teeth (SHED) could be induced to differentiate into functional vascular smooth muscle cells (vSMCs).
Methods: We utilized two cytokines of the TGF-β family, transforming growth factor beta 1 (TGF-β1) and bone morphogenetic protein 4 (BMP4), to induce SHED differentiation into SMCs. Quantitative real-time polymerase chain reaction (RT-qPCR) was used to assess mRNA expression, and protein expression was analyzed using flow cytometry, western blot and immunostaining. Additionally, to examine whether these SHED-derived SMCs possess the same function as primary SMCs, in vitro Matrigel angiogenesis assay, fibrin gel bead assay, and functional contraction study were used here.
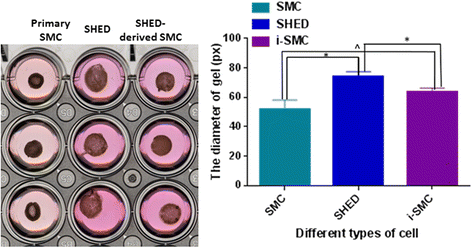
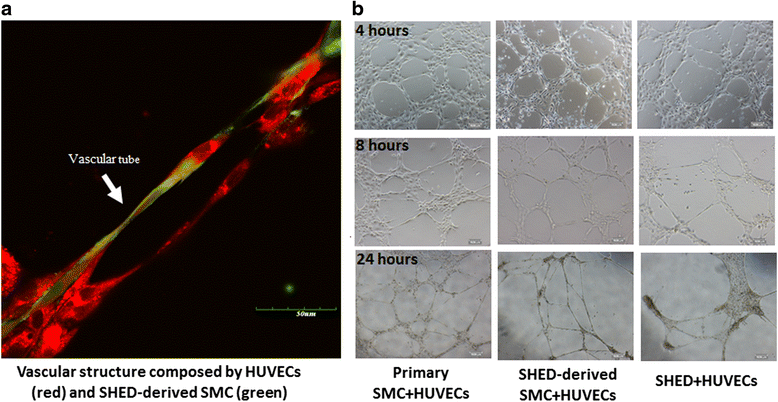
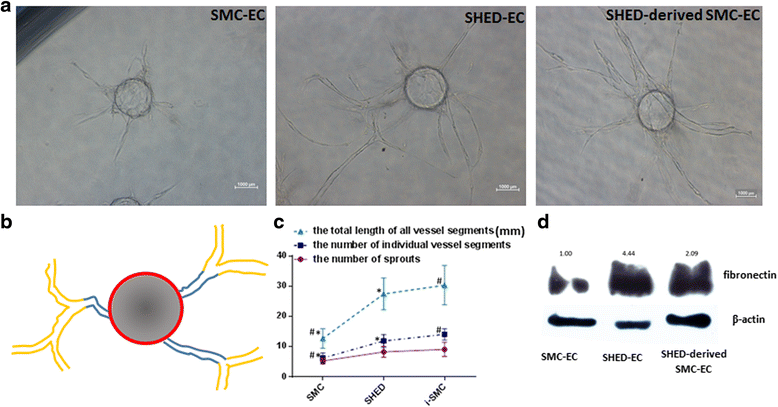
Results: By analyzing the expression of specific markers of SMCs (α-SMA, SM22α, Calponin, and SM-MHC), we confirmed that TGF-β1, and not BMP4, could induce SHED differentiation into SMCs. The differentiation efficiency was relatively high (α-SMA+ 86.1%, SM22α+ 93.9%, Calponin+ 56.8%, and SM-MHC+ 88.2%) as assessed by flow cytometry. In vitro Matrigel angiogenesis assay showed that the vascular structures generated by SHED-derived SMCs and human umbilical vein endothelial cells (HUVECs) were comparable to primary SMCs and HUVECs in terms of vessel stability. Fibrin gel bead assay showed that SHED-derived SMCs had a stronger capacity for promoting vessel formation compared with primary SMCs. Further analyses of protein expression in fibrin gel showed that cultures containing SHED-derived SMCs exhibited higher expression levels of Fibronectin than the primary SMCs group. Additionally, it was also confirmed that SHED-derived SMCs exhibited functional contractility. When SB-431542, a specific inhibitor of ALK5 was administered, TGF-β1 stimulation could not induce SHED into SMCs, indicating that the differentiation of SHED into SMCs is somehow related to the TGF-β1-ALK5 signaling pathway.
Conclusions: SHED could be successfully induced into functional SMCs for vascular tissue engineering, and this course could be regulated through the ALK5 signaling pathway. Hence, SHED appear to be a promising candidate cell type for vascular tissue engineering.
Keywords: Angiogenesis; Dental pulp stem cells; Smooth muscle cells; Stemness; Tissue engineering.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

