Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use
- PMID: 28115021
- PMCID: PMC5260040
- DOI: 10.1186/s13287-016-0465-2
Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use
Abstract
Background: Increasing evidence suggests the safety and efficacy of mesenchymal stromal cells (MSC) as advanced therapy medicinal products because of their immunomodulatory properties and supportive role in hematopoiesis. Although bone marrow remains the most common source for obtaining off-the-shelf MSC, cord blood (CB) represents an alternative source, which can be collected noninvasively and without major ethical concerns. However, the low estimated frequency and inconsistency of successful isolation represent open challenges for the use of CB-derived MSC in clinical trials. This study explores whether CB may represent a suitable source of MSC for clinical use and analyzes several in vitro parameters useful to better define the quality of CB-derived MSC prior to clinical application.
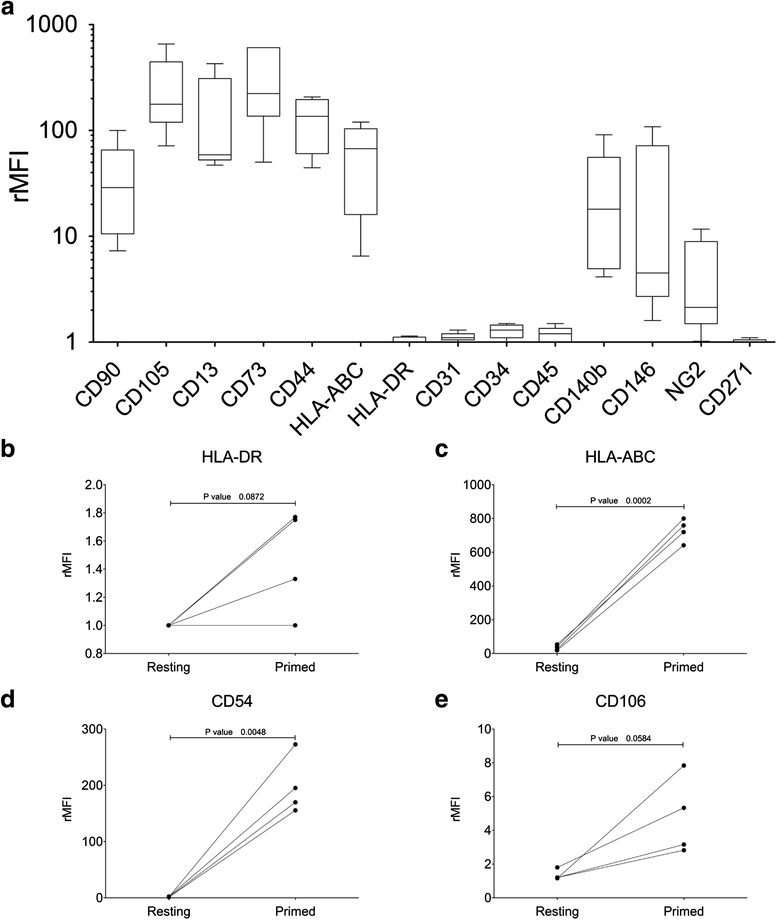
Methods: CB units (n = 50) selected according to quality criteria (CB volume ≥ 20 ml, time from collection ≤ 24 h) were cultured using a standardized procedure for CB-MSC generation. MSC were analyzed for their growth potential and secondary colony-forming capacity. Immunophenotype and multilineage differentiation potential of culture-expanded CB-MSC were assessed to verify MSC identity. The immunomodulatory activity at resting conditions and after inflammatory priming (IFN-γ-1b and TNF-α for 48 hours) was explored to assess the in vitro potency of CB-MSC prior to clinical application. Molecular karyotyping was used to assess the genetic stability after prolonged MSC expansion.
Results: We were able to isolate MSC colonies from 44% of the processed units. Our results do not support a role of CB volume in determining the outcome of the cultures, in terms of both isolation and proliferative capacity of CB-MSC. Particularly, we have confirmed the existence of two different CB-MSC populations named short- and long-living (SL- and LL-) CBMSC, clearly diverging in their growth capacity and secondary colony-forming efficiency. Only LL-CBMSC were able to expand consistently and to survive for longer periods in vitro, while preserving genetic stability. Therefore, they may represent interesting candidates for therapeutic applications. We have also observed that LL-CBMSC were not equally immunosuppressive, particularly after inflammatory priming and despite upregulating priming-inducible markers.
Conclusions: This work supports the use of CB as a potential MSC source for clinical applications, remaining more readily available compared to conventional sources. We have provided evidence that not all LL-CBMSC are equally immunosuppressive in an inflammatory environment, suggesting the need to include the assessment of potency among the release criteria for each CB-MSC batch intended for clinical use, at least for the treatment of immune disorders as GvHD.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

