Cyclic Boronates Inhibit All Classes of β-Lactamases
- PMID: 28115348
- PMCID: PMC5365654
- DOI: 10.1128/AAC.02260-16
Cyclic Boronates Inhibit All Classes of β-Lactamases
Abstract
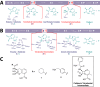
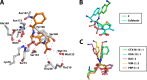
β-Lactamase-mediated resistance is a growing threat to the continued use of β-lactam antibiotics. The use of the β-lactam-based serine-β-lactamase (SBL) inhibitors clavulanic acid, sulbactam, and tazobactam and, more recently, the non-β-lactam inhibitor avibactam has extended the utility of β-lactams against bacterial infections demonstrating resistance via these enzymes. These molecules are, however, ineffective against the metallo-β-lactamases (MBLs), which catalyze their hydrolysis. To date, there are no clinically available metallo-β-lactamase inhibitors. Coproduction of MBLs and SBLs in resistant infections is thus of major clinical concern. The development of "dual-action" inhibitors, targeting both SBLs and MBLs, is of interest, but this is considered difficult to achieve due to the structural and mechanistic differences between the two enzyme classes. We recently reported evidence that cyclic boronates can inhibit both serine- and metallo-β-lactamases. Here we report that cyclic boronates are able to inhibit all four classes of β-lactamase, including the class A extended spectrum β-lactamase CTX-M-15, the class C enzyme AmpC from Pseudomonas aeruginosa, and class D OXA enzymes with carbapenem-hydrolyzing capabilities. We demonstrate that cyclic boronates can potentiate the use of β-lactams against Gram-negative clinical isolates expressing a variety of β-lactamases. Comparison of a crystal structure of a CTX-M-15:cyclic boronate complex with structures of cyclic boronates complexed with other β-lactamases reveals remarkable conservation of the small-molecule binding mode, supporting our proposal that these molecules work by mimicking the common tetrahedral anionic intermediate present in both serine- and metallo-β-lactamase catalysis.
Keywords: antibiotic resistance; beta-lactamases; beta-lactams; boronate; carbapenemase; inhibitors; metalloenzymes.
Copyright © 2017 Cahill et al.
Figures
References
-
- Versporten A, Bolokhovets G, Ghazaryan L, Abilova V, Pyshnik G, Spasojevic T, Korinteli I, Raka L, Kambaralieva B, Cizmovic L, Carp A, Radonjic V, Maqsudova N, Celik HD, Payerl-Pal M, Pedersen HB, Sautenkova N, Goossens H. 2014. Antibiotic use in Eastern Europe: a cross-national database study in coordination with the WHO regional office for Europe. Lancet Infect Dis 14:381–387. doi:10.1016/S1473-3099(14)70071-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

