Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells
- PMID: 28115366
- PMCID: PMC5374731
- DOI: 10.1182/blood-2016-09-692574
Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells
Abstract
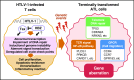
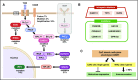
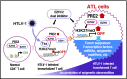
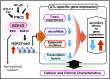
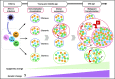
Adult T-cell leukemia (ATL) is an aggressive T-cell malignancy caused by human T-cell leukemia virus type 1 (HTLV-1) that develops through a multistep carcinogenesis process involving 5 or more genetic events. We provide a comprehensive overview of recently uncovered information on the molecular basis of leukemogenesis in ATL. Broadly, the landscape of genetic abnormalities in ATL that include alterations highly enriched in genes for T-cell receptor-NF-κB signaling such as PLCG1, PRKCB, and CARD11 and gain-of function mutations in CCR4 and CCR7 Conversely, the epigenetic landscape of ATL can be summarized as polycomb repressive complex 2 hyperactivation with genome-wide H3K27 me3 accumulation as the basis of the unique transcriptome of ATL cells. Expression of H3K27 methyltransferase enhancer of zeste 2 was shown to be induced by HTLV-1 Tax and NF-κB. Furthermore, provirus integration site analysis with high-throughput sequencing enabled the analysis of clonal composition and cell number of each clone in vivo, whereas multicolor flow cytometric analysis with CD7 and cell adhesion molecule 1 enabled the identification of HTLV-1-infected CD4+ T cells in vivo. Sorted immortalized but untransformed cells displayed epigenetic changes closely overlapping those observed in terminally transformed ATL cells, suggesting that epigenetic abnormalities are likely earlier events in leukemogenesis. These new findings broaden the scope of conceptualization of the molecular mechanisms of leukemogenesis, dissecting them into immortalization and clonal progression. These recent findings also open a new direction of drug development for ATL prevention and treatment because epigenetic marks can be reprogrammed. Mechanisms underlying initial immortalization and progressive accumulation of these abnormalities remain to be elucidated.
© 2017 by The American Society of Hematology.
Figures

References
-
- Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517-e526. - PubMed
-
- Watanabe T. Current status of HTLV-1 infection. Int J Hematol. 2011;94(5):430-434. - PubMed
-
- Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84(2):327-335. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

