Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma
- PMID: 28115578
- PMCID: PMC5464463
- DOI: 10.1093/neuonc/now287
Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma
Abstract
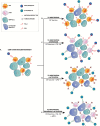
Background: Adaptive immune resistance in the tumor microenvironment appears to attenuate the immunotherapeutic targeting of glioblastoma (GBM). In this study, we identified a tumor-infiltrating myeloid cell (TIM) population that expands in response to dendritic cell (DC) vaccine treatment. The aim of this study was to understand how this programmed death ligand 1 (PD-L1)-expressing population restricts activation and tumor-cytolytic function of vaccine-induced tumor-infiltrating lymphocytes (TILs).
Methods: To test this hypothesis in our in vivo preclinical model, we treated mice bearing intracranial gliomas with DC vaccination ± murine anti-PD-1 monoclonal antibody (mAb) blockade or a colony stimulating factor 1 receptor inhibitor (CSF-1Ri) (PLX3397) and measured overall survival. We then harvested and characterized the PD-L1+ TIM population and its role in TIL activation and tumor cytolysis in vitro.
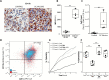
Results: Our data indicated that the majority of PD-L1 expression in the GBM environment is contributed by TIMs rather than by tumor cells themselves. While PD-1 blockade partially reversed the TIL dysfunction, targeting TIMs directly with CSF-1Ri altered TIM expression of key chemotactic factors associated with promoting increased TIL infiltration after vaccination. Neither PD-1 mAb nor CSF-1Ri had a demonstrable therapeutic benefit alone, but when combined with DC vaccination, a significant survival benefit was observed. When the tripartite regimen was given (DC vaccine, PD-1 mAb, PLX3397), long-term survival was noted together with an increase in the number of TILs and TIL activation.
Conclusion: Together, these studies elucidate the role that TIMs play in mediating adaptive immune resistance in the GBM microenvironment and provide evidence that they can be manipulated pharmacologically with agents that are clinically available. Development of immune resistance in response to active vaccination in GBM can be reversed with dual administration of CSF-1Ri and PD-1 mAb.
Keywords: CSF-1R; PD-1; cancer; checkpoint inhibitors; dendritic cell vaccine; glioblastoma; immunotherapy.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures




References
-
- Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. - PubMed
-
- Heimberger AB, Hussain SF, Aldape K, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. J Clin Oncol. 2006;24(18):107s.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

