Nickel pincer model of the active site of lactate racemase involves ligand participation in hydride transfer
- PMID: 28115700
- PMCID: PMC5307482
- DOI: 10.1073/pnas.1616038114
Nickel pincer model of the active site of lactate racemase involves ligand participation in hydride transfer
Abstract
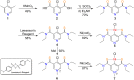
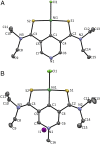
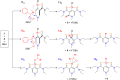
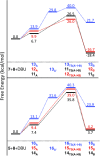
Lactate racemase is the first enzyme known to possess a metal pincer active site. The enzyme interconverts d- and l-lactic acid, which is important for the assembly of cell walls in many microorganisms. Here, we report a synthetic model of the active site of lactate racemase, which features a pyridinium-based SCS pincer ligand framework bound to nickel. The model complex mediates the dehydrogenation of alcohols, a reaction relevant to lactate racemization. Experimental and computational data indicate ligand participation in the dehydrogenation reaction.
Keywords: biomimetic chemistry; hydride transfer; lactate racemase; nickel; pincer ligands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Szabo KJ, Wendt OF. Pincer and Pincer-Type Complexes. Wiley-VCH; Weinheim: 2014.
-
- Morales-Morales D, Jensen CM. The Chemistry of Pincer Compounds. Elsevier; Amsterdam: 2007.
-
- Albrecht M, van Koten G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew Chem Int Ed Engl. 2001;40(20):3750–3781. - PubMed
-
- van der Boom ME, Milstein D. Cyclometalated phosphine-based pincer complexes: Mechanistic insight in catalysis, coordination, and bond activation. Chem Rev. 2003;103(5):1759–1792. - PubMed
-
- Desguin B, et al. A tethered niacin-derived pincer complex with a nickel-carbon bond in lactate racemase. Science. 2015;349(6243):66–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

