Superresolution imaging reveals activity-dependent plasticity of axon morphology linked to changes in action potential conduction velocity
- PMID: 28115721
- PMCID: PMC5307438
- DOI: 10.1073/pnas.1607541114
Superresolution imaging reveals activity-dependent plasticity of axon morphology linked to changes in action potential conduction velocity
Abstract
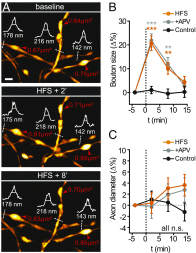
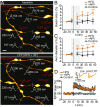
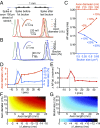
Axons convey information to nearby and distant cells, and the time it takes for action potentials (APs) to reach their targets governs the timing of information transfer in neural circuits. In the unmyelinated axons of hippocampus, the conduction speed of APs depends crucially on axon diameters, which vary widely. However, it is not known whether axon diameters are dynamic and regulated by activity-dependent mechanisms. Using time-lapse superresolution microscopy in brain slices, we report that axons grow wider after high-frequency AP firing: synaptic boutons undergo a rapid enlargement, which is mostly transient, whereas axon shafts show a more delayed and progressive increase in diameter. Simulations of AP propagation incorporating these morphological dynamics predicted bidirectional effects on AP conduction speed. The predictions were confirmed by electrophysiological experiments, revealing a phase of slowed down AP conduction, which is linked to the transient enlargement of the synaptic boutons, followed by a sustained increase in conduction speed that accompanies the axon shaft widening induced by high-frequency AP firing. Taken together, our study outlines a morphological plasticity mechanism for dynamically fine-tuning AP conduction velocity, which potentially has wide implications for the temporal transfer of information in the brain.
Keywords: STED microscopy; action potential conduction velocity; axons; plasticity; synaptic boutons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91(2):555–602. - PubMed
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311(5765):1290–1293. - PubMed
-
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nägerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60(4):590–597. - PubMed
-
- Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331(6017):599–601. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

