Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization
- PMID: 28115846
- PMCID: PMC5221810
- DOI: 10.2147/IJN.S114754
Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization
Abstract
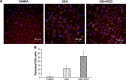
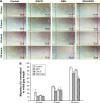
Neovascularization (NV) of the cornea can disrupt visual function, causing ocular diseases, including blindness. Therefore, treatment of corneal NV has a high public health impact. Epigalloccatechin-3-gallate (EGCG), presenting antiangiogenesis effects, was chosen as an inhibitor to treat human vascular endothelial cells for corneal NV treatment. An arginine-glycine-aspartic acid (RGD) peptide-hyaluronic acid (HA)-conjugated complex coating on the gelatin/EGCG self-assembly nanoparticles (GEH-RGD NPs) was synthesized for targeting the αvβ3 integrin on human umbilical vein endothelial cells (HUVECs) in this study, and a corneal NV mouse model was used to evaluate the therapeutic effect of this nanomedicine used as eyedrops. HA-RGD conjugation via COOH and amine groups was confirmed by 1H-nuclear magnetic resonance and Fourier-transform infrared spectroscopy. The average diameter of GEH-RGD NPs was 168.87±22.5 nm with positive charge (19.7±2 mV), with an EGCG-loading efficiency up to 95%. Images of GEH-RGD NPs acquired from transmission electron microscopy showed a spherical shape and shell structure of about 200 nm. A slow-release pattern was observed in the nanoformulation at about 30% after 30 hours. Surface plasmon resonance confirmed that GEH-RGD NPs specifically bound to the integrin αvβ3. In vitro cell-viability assay showed that GEH-RGD efficiently inhibited HUVEC proliferation at low EGCG concentrations (20 μg/mL) when compared with EGCG or non-RGD-modified NPs. Furthermore, GEH-RGD NPs significantly inhibited HUVEC migration down to 58%, lasting for 24 hours. In the corneal NV mouse model, fewer and thinner vessels were observed in the alkali-burned cornea after treatment with GEH-RGD NP eyedrops. Overall, this study indicates that GEH-RGD NPs were successfully developed and synthesized as an inhibitor of vascular endothelial cells with specific targeting capacity. Moreover, they can be used in eyedrops to inhibit angiogenesis in corneal NV mice.
Keywords: RGD peptide; antiangiogenesis; corneal neovascularization; epigallocatechin gallate (EGCG); hyaluronic acid (HA); vascular endothelial cells.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Huang TL, Hsu SY, Tsai RK, Sheu MM. Etiology of ocular diseases in elderly Amis aborigines in Eastern Taiwan (the Amis Eye Study) Jpn J Ophthalmol. 2010;54:266–271. - PubMed
-
- Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor: a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77. - PubMed
-
- Bock F, König Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–284. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

