Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization
- PMID: 28115848
- PMCID: PMC5221800
- DOI: 10.2147/IJN.S123062
Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization
Abstract
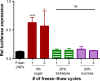
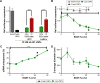
The broadest clinical application of siRNA therapeutics will be facilitated by drug-loaded delivery systems that maintain stability and potency for long times under ambient conditions. In the present study, we seek to better understand the stability and effect of storage conditions on lipidoid nanoparticles (LNPs), which have been previously shown by our group and others to potently deliver RNA to various cell and organ targets both in vitro and in vivo. Specifically, this study evaluates the influence of pH, temperature, and lyophilization on LNP efficacy in HeLa cells. When stored under aqueous conditions, we found that refrigeration (2°C) kept LNPs the most stable over 150 days compared to storage in the -20°C freezer or at room temperature. Because the pH of the storage buffer was not found to influence stability, it is suggested that the LNPs be stored under physiologically appropriate conditions (pH 7) for ease of use. Although aggregation and loss of efficacy were observed when LNPs were subjected to freeze-thaw cycles, their stability was retained with the use of the cryoprotectants, trehalose, and sucrose. Initially, lyophilization of the LNPs followed by reconstitution in aqueous buffer also led to reductions in efficacy, most likely due to aggregation upon reconstitution. Although the addition of ethanol to the reconstitution buffer restored efficacy, this approach is not ideal, as LNP solutions would require dialysis prior to use. Fortunately, we found that the addition of trehalose or sucrose to LNP solutions prior to lyophilization facilitated room temperature storage and reconstitution in aqueous buffer without diminishing delivery potency.
Keywords: lipid nanoparticles; lyophilization; lyoprotectants; nanoparticle stability; nanoparticle storage; siRNA delivery.
Conflict of interest statement
KAW reports two patents related to the lipidoid materials described in this work: US Patents 9,439,968 and 9,227,917. The authors report no further conflicts of interest in this work.
Figures



References
-
- Kasiewicz LN, Whitehead KA. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater. 2015;32:120–128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

