Radiotherapy and MVA-MUC1-IL-2 vaccine act synergistically for inducing specific immunity to MUC-1 tumor antigen
- PMID: 28116088
- PMCID: PMC5240430
- DOI: 10.1186/s40425-016-0204-3
Radiotherapy and MVA-MUC1-IL-2 vaccine act synergistically for inducing specific immunity to MUC-1 tumor antigen
Abstract
Background: We previously demonstrated that tumor irradiation potentiates cancer vaccines using genetic modification of tumor cells in murine tumor models. To investigate whether tumor irradiation augments the immune response to MUC1 tumor antigen, we have tested the efficacy of tumor irradiation combined with an MVA-MUC1-IL2 cancer vaccine (Transgene TG4010) for murine renal adenocarcinoma (Renca) cells transfected with MUC1.
Methods: Established subcutaneous Renca-MUC1 tumors were treated with 8 Gy radiation on day 11 and peritumoral injections of MVA-MUC1-IL2 vector on day 12 and 17, or using a reverse sequence of vaccine followed by radiation. Growth delays were monitored by tumor measurements and histological responses were evaluated by immunohistochemistry. Specific immunity was assessed by challenge with Renca-MUC1 cells. Generation of tumor-specific T cells was detected by IFN-γ production from splenocytes stimulated in vitro with tumor lysates using ELISPOT assays.
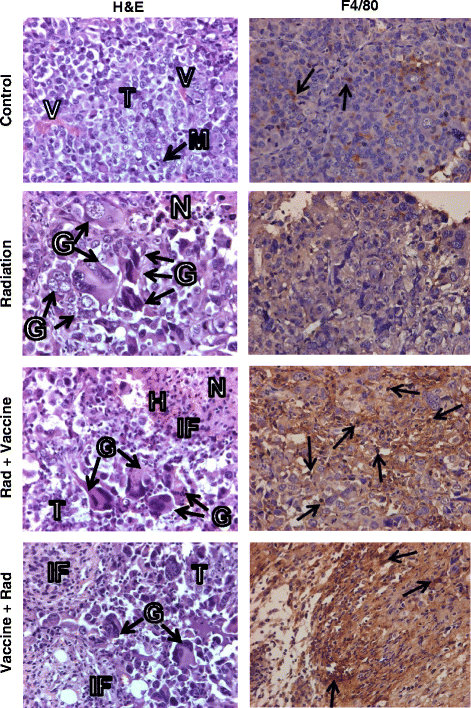
Results: Tumor growth delays observed by tumor irradiation combined with MVA-MUC1-IL-2 vaccine were significantly more prolonged than those observed by vaccine, radiation, or radiation with MVA empty vector. The sequence of cancer vaccine followed by radiation two days later resulted in 55-58% complete responders and 60% mouse long-term survival. This sequence was more effective than that of radiation followed by vaccine leading to 24-30% complete responders and 30% mouse survival. Responding mice were immune to challenge with Renca-MUC1 cells, indicating the induction of specific tumor immunity. Histology studies of regressing tumors at 1 week after therapy, revealed extensive tumor destruction and a heavy infiltration of CD45+ leukocytes including F4/80+ macrophages, CD8+ cytotoxic T cells and CD4+ helper T cells. The generation of tumor-specific T cells by combined therapy was confirmed by IFN-γ secretion in tumor-stimulated splenocytes. An abscopal effect was measured by rejection of an untreated tumor on the contralateral flank to the tumor treated with radiation and vaccine.
Conclusions: These findings suggest that cancer vaccine given prior to local tumor irradiation augments an immune response targeted at tumor antigens that results in specific anti-tumor immunity. These findings support further exploration of the combination of radiotherapy with cancer vaccines for the treatment of cancer.
Keywords: IL-2; MUC1; MVA vector; Radiation; Renal Cell Carcinoma.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
