The Development and Current Use of BCL-2 Inhibitors for the Treatment of Chronic Lymphocytic Leukemia
- PMID: 28116634
- PMCID: PMC5862560
- DOI: 10.1007/s11899-017-0359-0
The Development and Current Use of BCL-2 Inhibitors for the Treatment of Chronic Lymphocytic Leukemia
Abstract
The BCL-2 family of proteins integrates pro- and anti-apoptotic signals within the cell and is responsible for initiation of caspase-dependent apoptosis. Chronic lymphocytic leukemia (CLL) cells are particularly dependent on the anti-apoptotic protein BCL-2 for their survival, making this an attractive therapeutic target in CLL. Several early efforts to create inhibitors of the anti-apoptotic family members faced significant challenges, but eventually, the BCL-2 specific inhibitor venetoclax moved forward in CLL. Overall and complete response rates to venetoclax monotherapy in relapsed, refractory CLL are approximately 80 and 20%, respectively, even in patients with high-risk 17p deletion. Toxicities have been manageable and include neutropenia, diarrhea, and nausea. The risk of tumor lysis syndrome (TLS), seen in early experience with the drug, has been mitigated by the use of appropriate TLS risk assessment, prophylaxis, and management. Future studies of venetoclax will focus on combination approaches, predictive biomarker discovery, and mechanisms of resistance.
Keywords: BCL-2 inhibitors; Chronic lymphocytic leukemia; Clinical trials; Venetoclax.
Figures
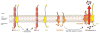
References
-
- Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109. - PubMed
-
- Pugazhenthi S, Nesterova A, Sable C, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275(15):10761–10766. - PubMed
-
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

