A Facile, Improved Synthesis of Sildenafil and Its Analogues
- PMID: 28117311
- PMCID: PMC5064236
- DOI: 10.3390/scipharm84030447
A Facile, Improved Synthesis of Sildenafil and Its Analogues
Abstract
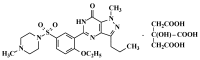
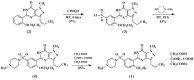
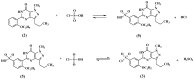
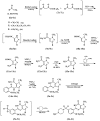
This paper describes the facile synthesis of sildenafil citrate (1) and its related compounds through an improved chlorosulfonation reaction using chlorosulfonic acid and thionyl chloride. This synthesis results in pure sildenafil with high yield. The structures of the compounds were determined with infrared (IR), ¹H-NMR and 13C-NMR spectroscopic methods, and high resolution mass spectroscopy (HRMS) analysis.
Keywords: chlorosulfonation; novel analogues; sildenafil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Anastasas P.T., Warner J.C. Green Chemistry: Theory and Practice. Oxford University Press; New York, NY, USA: 1988.
-
- Constable D.J.C., Curzons A.D., Cunningham V.L. Metrics to ‘green’ chemistry—which are the best? Green Chem. 2002;4:521–527. doi: 10.1039/B206169B. - DOI
-
- Andrew S.B., David B., Nicholas K.T. Pyrazolopyrimidinone Antianginal Agents. 5,250,534. U.S. Patent. 1993 Oct 5;
-
- Spryskov A.A., Kuzmina Y.L. Sulfonation reaction. XLVI. Equilibrium between toluenetrisulfonic acid and its trichloride. Russ. J. Gen. Chem. 1958;28:184–187.
LinkOut - more resources
Full Text Sources
Other Literature Sources