Tumour-associated macrophages as treatment targets in oncology
- PMID: 28117416
- PMCID: PMC5480600
- DOI: 10.1038/nrclinonc.2016.217
Tumour-associated macrophages as treatment targets in oncology
Abstract
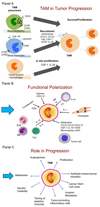
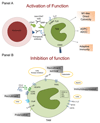
Macrophages are crucial drivers of tumour-promoting inflammation. Tumour-associated macrophages (TAMs) contribute to tumour progression at different levels: by promoting genetic instability, nurturing cancer stem cells, supporting metastasis, and taming protective adaptive immunity. TAMs can exert a dual, yin-yang influence on the effectiveness of cytoreductive therapies (chemotherapy and radiotherapy), either antagonizing the antitumour activity of these treatments by orchestrating a tumour-promoting, tissue-repair response or, instead, enhancing the overall antineoplastic effect. TAMs express molecular triggers of checkpoint proteins that regulate T-cell activation, and are targets of certain checkpoint-blockade immunotherapies. Other macrophage-centred approaches to anticancer therapy are under investigation, and include: inhibition of macrophage recruitment to, and/or survival in, tumours; functional re-education of TAMs to an antitumour, 'M1-like' mode; and tumour-targeting monoclonal antibodies that elicit macrophage-mediated extracellular killing, or phagocytosis and intracellular destruction of cancer cells. The evidence supporting these strategies is reviewed herein. We surmise that TAMs can provide tools to tailor the use of cytoreductive therapies and immunotherapy in a personalized medicine approach, and that TAM-focused therapeutic strategies have the potential to complement and synergize with both chemotherapy and immunotherapy.
Figures

References
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. - PubMed
-
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

