Sodium Chloride Enhances Recombinant Adeno-Associated Virus Production in a Serum-Free Suspension Manufacturing Platform Using the Herpes Simplex Virus System
- PMID: 28117600
- PMCID: PMC5346630
- DOI: 10.1089/hgtb.2016.151
Sodium Chloride Enhances Recombinant Adeno-Associated Virus Production in a Serum-Free Suspension Manufacturing Platform Using the Herpes Simplex Virus System
Abstract
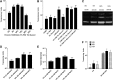
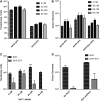
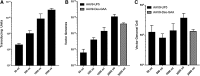
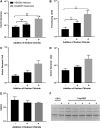
The increase in effective treatments using recombinant adeno-associated viral (rAAV) vectors has underscored the importance of scalable, high-yield manufacturing methods. Previous work from this group reported the use of recombinant herpes simplex virus type 1 (rHSV) vectors to produce rAAV in adherent HEK293 cells, demonstrating the capacity of this system and quality of the product generated. Here we report production and optimization of rAAV using the rHSV system in suspension HEK293 cells (Expi293F) grown in serum and animal component-free medium. Through adjustment of salt concentration in the medium and optimization of infection conditions, titers greater than 1 × 1014 vector genomes per liter (VG/liter) were observed in purified rAAV stocks produced in Expi293F cells. Furthermore, this system allowed for high-titer production of multiple rAAV serotypes (2, 5, and 9) as well as multiple transgenes (green fluorescent protein and acid α-glucosidase). A proportional increase in vector production was observed as this method was scaled, with a final 3-liter shaker flask production yielding an excess of 1 × 1015 VG in crude cell harvests and an average of 3.5 × 1014 total VG of purified rAAV9 material, resulting in greater than 1 × 105 VG/cell. These results support the use of this rHSV-based rAAV production method for large-scale preclinical and clinical vector production.
Keywords: AAV vectors production; gene therapy; herpes simplex virus; suspension cell lines.
Conflict of interest statement
M.P. declares no conflict of interest. L.A.-S., B.J.B., and N.C. have a pending patent application related to this research. B.J.B. is an inventor of intellectual property owned by the Johns Hopkins University and the University of Florida related to this research and has AAV patents licensed to various biopharmaceutical companies.
Figures
References
-
- Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014;1:427–451 - PubMed
-
- Hildinger M, Baldi L, Stettler M, et al. . High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol Lett 2007;29:1713–1721 - PubMed
-
- Durocher Y, Pham PL, St-Laurent G, et al. . Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J Virol Methods 2007;144:32–40 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
