Direct assessment of substrate binding to the Neurotransmitter:Sodium Symporter LeuT by solid state NMR
- PMID: 28117663
- PMCID: PMC5262378
- DOI: 10.7554/eLife.19314
Direct assessment of substrate binding to the Neurotransmitter:Sodium Symporter LeuT by solid state NMR
Abstract
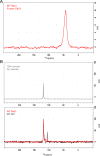
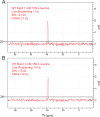
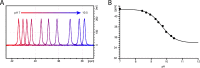
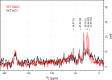
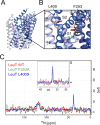
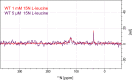
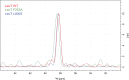
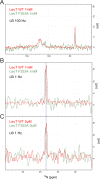
The Neurotransmitter:Sodium Symporters (NSSs) represent an important class of proteins mediating sodium-dependent uptake of neurotransmitters from the extracellular space. The substrate binding stoichiometry of the bacterial NSS protein, LeuT, and thus the principal transport mechanism, has been heavily debated. Here we used solid state NMR to specifically characterize the bound leucine ligand and probe the number of binding sites in LeuT. We were able to produce high-quality NMR spectra of substrate bound to microcrystalline LeuT samples and identify one set of sodium-dependent substrate-specific chemical shifts. Furthermore, our data show that the binding site mutants F253A and L400S, which probe the major S1 binding site and the proposed S2 binding site, respectively, retain sodium-dependent substrate binding in the S1 site similar to the wild-type protein. We conclude that under our experimental conditions there is only one detectable leucine molecule bound to LeuT.
Keywords: LeuT; aquifex aeolicus; biophysics; neuroscience; neurotransmitter transporters; solid state NMR; structural biology; substrate binding site.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Billesbølle CB, Krüger MB, Shi L, Quick M, Li Z, Stolzenberg S, Kniazeff J, Gotfryd K, Mortensen JS, Javitch JA, Weinstein H, Loland CJ, Gether U. Substrate-induced unlocking of the inner gate determines the catalytic efficiency of a neurotransmitter:sodium symporter. Journal of Biological Chemistry. 2015;290:26725–26738. doi: 10.1074/jbc.M115.677658. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

