Novel adverse outcome pathways revealed by chemical genetics in a developing marine fish
- PMID: 28117666
- PMCID: PMC5302885
- DOI: 10.7554/eLife.20707
Novel adverse outcome pathways revealed by chemical genetics in a developing marine fish
Abstract
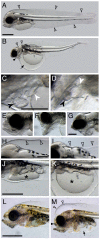
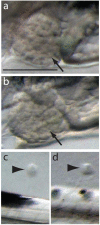
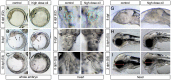
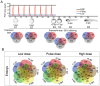
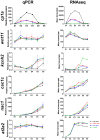
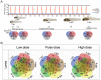
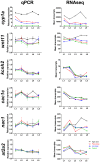
Crude oil spills are a worldwide ocean conservation threat. Fish are particularly vulnerable to the oiling of spawning habitats, and crude oil causes severe abnormalities in embryos and larvae. However, the underlying mechanisms for these developmental defects are not well understood. Here, we explore the transcriptional basis for four discrete crude oil injury phenotypes in the early life stages of the commercially important Atlantic haddock (Melanogrammus aeglefinus). These include defects in (1) cardiac form and function, (2) craniofacial development, (3) ionoregulation and fluid balance, and (4) cholesterol synthesis and homeostasis. Our findings suggest a key role for intracellular calcium cycling and excitation-transcription coupling in the dysregulation of heart and jaw morphogenesis. Moreover, the disruption of ionoregulatory pathways sheds new light on buoyancy control in marine fish embryos. Overall, our chemical-genetic approach identifies initiating events for distinct adverse outcome pathways and novel roles for individual genes in fundamental developmental processes.
Keywords: Atlantic haddock; cardiac abnormalities; chemical genetics; craniofacial abnormalities; crude oil; ecology; evolutionary biology; genomics; transcriptome.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






Similar articles
-
Urban stormwater and crude oil injury pathways converge on the developing heart of a shore-spawning marine forage fish.Aquat Toxicol. 2020 Dec;229:105654. doi: 10.1016/j.aquatox.2020.105654. Epub 2020 Oct 11. Aquat Toxicol. 2020. PMID: 33161306
-
Crude oil exposures reveal roles for intracellular calcium cycling in haddock craniofacial and cardiac development.Sci Rep. 2016 Aug 10;6:31058. doi: 10.1038/srep31058. Sci Rep. 2016. PMID: 27506155 Free PMC article.
-
Co-exposure to UV radiation and crude oil increases acute embryotoxicity and sublethal malformations in the early life stages of Atlantic haddock (Melanogrammus aeglefinus).Sci Total Environ. 2023 Feb 10;859(Pt 1):160080. doi: 10.1016/j.scitotenv.2022.160080. Epub 2022 Nov 11. Sci Total Environ. 2023. PMID: 36375555
-
The influence of heart developmental anatomy on cardiotoxicity-based adverse outcome pathways in fish.Aquat Toxicol. 2016 Aug;177:515-25. doi: 10.1016/j.aquatox.2016.06.016. Epub 2016 Jun 21. Aquat Toxicol. 2016. PMID: 27447099 Review.
-
Effects of petrogenic pollutants on North Atlantic and Arctic Calanus copepods: From molecular mechanisms to population impacts.Aquat Toxicol. 2024 Feb;267:106825. doi: 10.1016/j.aquatox.2023.106825. Epub 2023 Dec 28. Aquat Toxicol. 2024. PMID: 38176169 Review.
Cited by
-
Oil droplet fouling and differential toxicokinetics of polycyclic aromatic hydrocarbons in embryos of Atlantic haddock and cod.PLoS One. 2017 Jul 5;12(7):e0180048. doi: 10.1371/journal.pone.0180048. eCollection 2017. PLoS One. 2017. PMID: 28678887 Free PMC article.
-
Atlantic cod (Gadus morhua) embryos are highly sensitive to short-term 3,4-dichloroaniline exposure.Toxicol Rep. 2021 Oct 11;8:1754-1761. doi: 10.1016/j.toxrep.2021.10.006. eCollection 2021. Toxicol Rep. 2021. PMID: 34703771 Free PMC article.
-
Brazilian silverside, Atherinella brasiliensis (Quoy & Gaimard,1825) embryos as a test-species for marine fish ecotoxicological tests.PeerJ. 2021 Apr 14;9:e11214. doi: 10.7717/peerj.11214. eCollection 2021. PeerJ. 2021. PMID: 33954044 Free PMC article.
-
Embryonic Crude Oil Exposure Impairs Growth and Lipid Allocation in a Keystone Arctic Forage Fish.iScience. 2019 Sep 27;19:1101-1113. doi: 10.1016/j.isci.2019.08.051. Epub 2019 Aug 30. iScience. 2019. PMID: 31536959 Free PMC article.
-
Adult killifish exposure to crude oil perturbs embryonic gene expression and larval morphology in first- and second-generation offspring.bioRxiv [Preprint]. 2025 Feb 15:2025.02.10.637481. doi: 10.1101/2025.02.10.637481. bioRxiv. 2025. PMID: 39990372 Free PMC article. Preprint.
References
-
- Adams J, Bornstein JM, Munno K, Hollebone B, King T, Brown RS, Hodson PV. Identification of compounds in heavy fuel oil that are chronically toxic to rainbow trout embryos by effects-driven chemical fractionation. Environmental Toxicology and Chemistry. 2014;33:825–835. doi: 10.1002/etc.2497. - DOI - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 2010;29:730–741. doi: 10.1002/etc.34. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

